Abstract
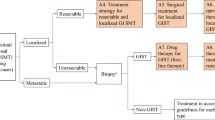
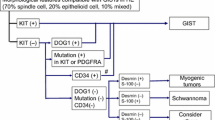
Diagnostic and treatment strategies for gastrointestinal stromal tumors (GISTs) have evolved greatly since the introduction of molecularly targeted therapies. Although several clinical practice guidelines are extant, such as those published by the National Comprehensive Cancer Network and the European Society of Medical Oncology, it is not clear as to whether these are appropriate for clinical practice in Japan. Therefore, clinical practice guidelines for the optimal diagnosis and treatment of GIST tailored for the Japanese situation have often been requested. For this reason, the Japanese Clinical Practice Guideline for GIST was proposed by the GIST Guideline Subcommittee, with the official approval of the Clinical Practice Guidelines Committee for Cancer of the Japan Society of Clinical Oncology (JSCO), and was published after assessment by the Guideline Evaluation Committee of JSCO. The GIST Guideline Subcommittee consists of members from JSCO, the Japanese Gastric Cancer Association (JGCA), and the Japanese Study Group on GIST, with the official approval of these organizations. The GIST Guideline Subcommittee is not influenced by any other organizations or third parties. Revision of the guideline may be done periodically, with the approval of the GIST Guideline Subcommittee, either every 3 years or when important new evidence that might alter the optimal diagnosis and treatment of GIST emerges. Here we present the English version of the Japanese Clinical Practice Guideline for GIST prepared by the GIST Guideline Subcommittee.
Similar content being viewed by others
References
Demetri GD, Benjamin RS, Blanke CD, et al; NCCN Task Force (2007) NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST) — update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5(Suppl 2): S1–S29
Blay JY, Bonvalot S, Casali P, et al. (2005) Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST consensus conference of 20–21 March 2004, under the auspices of ESMO. Ann Oncol 16:566–578
Kubota T (2006) Gastrointestinal stromal tumor (GIST) and imatinib. Int J Clin Oncol 11:184–189
The GIST Guideline Subcommittee of the Clinical Practice Guideline Committee for Cancer of JSCO (eds) (2008) Japanese clinical practice guidelines for GIST (1st edn). Kanehara, Tokyo, http://jsco-cpg.jp/item/03/index.html
Hirota S, Isozaki K, Moriyama Y, et al. (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577–580
Miettinen M, Sobin LH, Sarlomo-Rikala M. (2000) Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with reference to CD117 (KIT). Mod Pathol 13:1134–1142
Heinrich MC, Corless CL, Duensing A, et al. (2003) PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299:708–710
Hirota S, Ohashi A, Nishida T, et al. (2003) Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumors. Gastroenterology 125:660–667
Pauls K, Merkelbach-Bruse S, Thal D, et al. (2005) PDGFRalpha-and c-kit-mutated gastrointestinal stromal tumours (GISTs) are characterized by distinctive histological and immunohistochemical features. Histopathology 46:166–175
Sihto H, Sarlomo-Rikala M, Tynninen O, Tanner M, et al. (2005) KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol 23:49–57
Heinrich MC, Corless CL, Demetri GD, et al. (2003) Kinase mutation and imatinib response in patients with metastatic gastrointestinal stromal tumors. J Clin Oncol 21:4342–4349
Tarni C, Merkel E, Canutescu AA, et al. (2005) Analysis of KIT mutations in sporadic and familial gastrointestinal stromal tumors: therapeutic implications through protein modeling. Clin Cancer Res 11:3668–3677
Fletcher CD, Berman JJ, Corless C, et al. (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33:459–465
Wong NACS, Young R, Malcomson RDG, et al. (2000) Prognostic indicators for gastrointestinal stromal tumours: a clinicopathological and immunohistochemical study of 108 resected cases of the stomach. Histopathology 47:2247–2253
Singer S, Rubin BP, Lux ML, et al. (2002) Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 20:3898–3905
Fujimoto Y, Nakanishi Y, Yoshimura K, Shimoda T (2003) Clinicopathologic study of primary malignant gastrointestinal tumor of the stomach, with special reference to prognostic factors: analysis of results in 140 surgically resected patients. Gastric Cancer 6:39–48
Hasegawa T, Matsuno Y, Shimoda T, Hirohashi S (2002) Gastrointestinal stromal tumor: consistent CD117 immunostaining for diagnosis, and prognostic classification based on tumor size and MIB-1 grade. Hum Pathol 33:669–676
Jhala NC, Jhala D, Eltoum I, et al. (2004) Endoscopic ultrasoundguided fine-needle aspiration biopsy: a powerful tool to obtain samples from small lesions. Cancer 102:239–246
Arima M, Harada N, Kozu T, et al. (1999) Endoscopic ultrasonography-guided fine needle aspiration biopsy for upper gastrointestinal diseases. Endoscopia Digestiva 11:91–100
Tateishi U, Hasegawa T, Satake M, et al. (2003) Gastrointestinal stromal tumor: correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr 27:792–798
Kim HC, Lee JM, Kim KW, et al. (2004) Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol 183:893–898
Choi H, Charnsangavej C, de Castro Faria S, et al. (2004) CT Evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 183:1619–1628
Antoch G, Kanja J, Bauer S, et al. (2002) Comparison of PET, CT and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumor. J Nucl Med 45:357–365
Gayed I, Vu T, Iyer R, et al. (2004) The role of 18FDG-PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med 45:17–21
Shanker S, van Sonnenberg E, Desai J, et al. (2005) Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology 235: 892–898
DeMatteo RP, Lewis JJ, Leung D, et al. (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Fong Y, Coit DG, Woodruff JM, et al. (1993) Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 217: 72–77
DeMatteo RP, Heinrich MC, El-Rifai WM, Demetri G (2002) Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 33:466–477
Nguyen SQ, Divino CM, Wang JL, Dikman SH (2006) Laparoscopic management of gastrointestinal stromal tumors. Surg Endosc 20:713–716
Otani Y, Furukawa T, Yoshida M, et al. (2006) Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 139:484–492
Novitsky YW, Kercher KW, Sing RF, et al. (2006) Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 243:738–745
Nishimura J, Nakajima K, Omori T, et al. (2007) Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs open resection. Surg Endosc 21:875–878
Takahashi T, Nakajima K, Nishitani A, et al. (2007) An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol 12:369–374
van Oosterom AT, Judson I, Verweij J, et al. (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358:1421–1423
Demetri GD, Von Mehren M, Blanke CD, et al. (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480
Verweij J, Casali PG, Zalcberg J, et al. (2004) Progression-free survival in gastrointestinal stromal tumors with high-dose imatinib: randomized trial. Lancet 364:1127–1134
Blanke CD, Rankin C, Demetri GD, et al. (2008) Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 26:626–632
Zalcberg JR, Verweij J, Casali PG, et al. (2005) Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 41:1751–1757
Blay JY, Le Cesne A, Ray-Coquard I, et al. (2007) Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 25:1107–1113
Heinrich MC, Corless CL, Blanke CD, et al. (2006) Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 24:4764–4774
Demetri GD, van Oosterom AT, Garrett CR, et al. (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338
Z9001. A phase III randomized double-blind study of adjuvant STI571 (Gleevec™) versus placebo in patients following the resection of primary gastrointestinal stromal tumor (GIST). Available at: https://www.acosog.org/announcements/ACOSOG_Z9001A4_ Protocol_20050601.pdf
Bauer S, Hartmann JT, de Wit M, et al. (2005) Resection of residual disease in patients with metastatic gastrointestinal stromal tumors responding to treatment with imatinib. Int J Cancer 117:316–325
Raut CP, Posner M, Desai J, et al. (2006) Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 24:2325–2331
Rutkowski P, Nowecki Z, Nyckowski P, et al. (2006) Surgical treatment of patients with initially inoperable and/or metastatic gastrointestinal stromal tumors (GIST) during therapy with imatinib mesylate. J Surg Oncol 93:304–311
DeMatteo RP, Maki RG, Singer S, et al. (2007) Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 245:347–352
Van Glabbeke M, Verweij J, Casali PG, et al. (2005) Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 23:5795–5804
Gronchi A, Fiore M, Miselli F, et al. (2007) Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 245:341–346
Hasegawa J, Kanda T, Hirota S, et al. (2007) Surgical interventions for focal progression of advanced gastrointestinal stromal tumors under imatinib therapy. Int J Clin Oncol 12:212–217
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nishida, T., Hirota, S., Yanagisawa, A. et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 13, 416–430 (2008). https://doi.org/10.1007/s10147-008-0798-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-008-0798-7