Abstract
Perioperative anaphylaxis is a severe adverse event during anesthesia that requires prompt diagnosis and treatment by physicians, including anesthesiologists. Muscle relaxants and antibiotics are the most common drugs that cause perioperative anaphylaxis in Japan, as in many countries. In addition, sugammadex appears to be a primary causative agent. Obtaining previous anesthesia records is necessary in a patient with a history of allergic reactions during anesthesia, whenever possible, to avoid recurrence of anaphylaxis. Although medical staff are likely to notice abnormal vital signs because of complete monitoring during anesthesia, surgical drapes make it difficult to notice the appearance of skin symptoms. Even if there are no skin symptoms, anaphylaxis should be suspected, especially when hypotension resistant to inotropes and vasopressors persists. For improving the diagnostic accuracy of anaphylaxis, it is helpful to collect blood samples to measure histamine/tryptase concentrations immediately after the events and at baseline. The first-line treatment for anaphylaxis is adrenaline. In the perioperative setting, adrenaline should be administered through the intravenous route, which has a faster effect onset and is secured in most cases. Adrenaline can cause serious complications including severe arrhythmias if the appropriate dose is not selected according to the severity of symptoms. The anesthesiologist should identify the causative agent after adverse events. The gold standard for identifying the causative agent is the skin test, but in vitro tests including specific IgE antibody measurements and basophil activation tests are also beneficial. The Working Group of the Japanese Society of Anesthesiologists has developed this practical guide to help appropriate prevention, early diagnosis and treatment, and postoperative diagnosis of anaphylaxis during anesthesia.
Grade of recommendations and levels of evidence Anaphylaxis is a relatively rare condition with few controlled trials, and thus a so-called evidence-based scrutiny is difficult. Therefore, rather than showing evidence levels and indicating the level of recommendation, this practical guideline only describes the results of research available to date. The JSA will continue to investigate anaphylaxis during anesthesia, and the results may lead to an amendment of this practical guideline.
Similar content being viewed by others
Introduction
The Working Group of the Japanese Society of Anesthesiologists (JSA) developed this practical guide aimed to help appropriate prevention, early diagnosis and treatment, and postoperative diagnosis of anaphylaxis during anesthesia.
Guidelines in different countries define anaphylaxis, but they are generally similar [1,2,3,4,5,6]. For example, the Japanese Society of Allergy defines anaphylaxis as “a hypersensitivity reaction that can cause systemic allergic symptoms in multiple organs due to invasion by allergens and can be life threatening” [7]. Anaphylactic shock is defined as “anaphylaxis accompanied by decreased blood pressure or impaired consciousness” [7]. Since anaphylaxis elicits acute systemic hypersensitivity upon administration of the causative drug, prompt and appropriate treatment is necessary. Drugs used during the perioperative period include those with a high frequency of anaphylaxis. Anesthesiologists must be proficient in the diagnosis and treatment of anaphylaxis.
Anaphylaxis during anesthesia is not easy to diagnose due to the patient's unconsciousness and the large number of drugs that affect circulation and respiration. Patients are often covered with surgical drapes, which also delays identification of skin symptoms that are characteristic of anaphylaxis. Anesthesiologists must be aware of the occurrence of anaphylaxis in the operating room.
Anaphylaxis is classified into immunological anaphylaxis and non-immunological anaphylaxis. Immunological anaphylaxis is further divided into immunoglobulin (Ig) E-mediated anaphylaxis and non-IgE (IgG-mediated and immune complex-mediated) anaphylaxis [1]. Non-specific reactions that do not involve an immunological mechanism are referred to as non-immunologic anaphylaxis, and the conventional term “anaphylactoid reactions” is no longer used. Treatment for anaphylaxis is the same regardless of the underlying mechanism.
The key drug for treating anaphylaxis is adrenaline. The route and dose of adrenaline vary depending on the guideline. In this practical guide, intravenous administration is considered to be the default, because most patients are placed under anesthesia, and the intravenous route is thus already secured.
Anesthesiologists should be deeply involved in identifying the causative agent after the occurrence of anaphylaxis to prevent recurrence. The gold standard for identifying the causative agent is the skin test, but an in vitro test, the basophil activation test (BAT), is also promising [8]. Additionally, anesthesiologists should be cautious when anesthetizing patients with a history of anaphylaxis. This practical guide will be helpful to many anesthesiologists, because it covers a wide range of topics that anesthesiologists should familiarize themselves with to address perioperative anaphylaxis.
Epidemiology
Summary statement
-
Although the reported incidence of perioperative anaphylaxis varies depending on the study, many reports indicate it to be about 1 case in 10,000.
-
In many countries, muscle relaxants and antibiotics are the most common drugs that cause perioperative anaphylaxis. In addition to these drugs, anaphylaxis due to sugammadex appears to be common in Japan.
The frequency of anaphylaxis during anesthesia varies from study to study, ranging from 1 in 1250 to 1 in 18,600 [9,10,11,12,13,14]. Studies with low-frequency reports may have overlooked several cases of anaphylaxis and thus underreported their incidence. There are few studies on perioperative anaphylaxis in Japan, with the last one published 29 years ago. According to the report, the frequency of anaphylaxis was 0.01% or 1 in 10,000 cases, and the mortality rate was 4.76% [15].
Recently, a multicenter study on the epidemiology of perioperative anaphylaxis in Japan was conducted. The causes of the 67 cases of anaphylaxis were reported to be as follows: sugammadex in 15 cases, 29% of the total; rocuronium in 14 cases, 27%; cefazolin in 8 cases, 16%; antimicrobials other than cefazolin in 7 cases, 14%; and other causes in 7 cases, 14%. Thus, the top three drugs, sugammadex, rocuronium, and cefazolin, accounted for more than 70% of all causes [16].
Similarly, epidemiological studies of perioperative anaphylaxis have been conducted in several countries overseas. The most extensive study is the 6th National audit project (NAP6), which was conducted in the United Kingdom with the participation of all hospitals belonging to the National Health Service. Approximately 3 million general anesthesia cases performed in 2016 at hospitals participating in the study were included in the survey [17]. In NAP6, 541 suspected cases of anaphylaxis were included, of which 266 were concluded to be probable actual anaphylaxis. The frequency of anaphylaxis was about 1 in 12,000 cases. Upon analyzing the cases in which the causative agent was known, the most common cause was found to be antimicrobial agents in 94 cases, accounting for 47% of causative agents. This was followed by muscle relaxants in 65 cases (33%) and chlorhexidine in 18 cases (9%). Muscle relaxants and antibiotics were also the top causative agents in the UK [17]. A closer look at the causative agents identified by NAP6 reveals that they differ from the results of the Japanese survey. For example, the most common antibacterial drug that caused anaphylaxis was amoxicillin clavulanic acid, which is not used in Japan during the perioperative period. Not a single case of anaphylaxis due to cefazolin, the most common drug in Japan, occurred. The most common cause of anaphylaxis due to muscle relaxants was rocuronium, as in Japan; anaphylaxis due to atracurium and succinylcholine also occurred in a significant number of cases. Additionally, only one case of anaphylaxis due to sugammadex was reported [17].
According to a French analysis of the causative drugs of 692 anaphylaxis cases occurring during anesthesia, the causative drugs were muscle relaxants (61.6%), latex (16.6%), antibiotics (8.3%), intravenous anesthetics (5.1%), colloids (3.1%), narcotics (2.7%), and others (2.6%) [18]. Among the severe anaphylaxis cases due to muscle relaxants, independent risk factors associated with a fatal outcome were being male, emergency surgery, history of hypertension, cardiovascular diseases, obesity, and use of β-blockers. [19]. Nationwide epidemiological studies on perioperative anaphylaxis have not been conducted in Japan for a long time. Since there are differences in the drugs used during the perioperative period between Japan and other countries, large-scale epidemiological studies must be conducted in Japan in the future.
Preoperative diagnosis
Summary statement
-
Patients who have had an allergic reaction during previous anesthesia are at risk for anaphylaxis.
-
Patients with atopic dermatitis or history of allergies to drugs other than anesthetics do not need to be tested for drugs or products used during anesthesia.
-
If a patient had an allergic reaction during previous anesthesia, previous anesthesia records whenever possible must be obtained.
-
In emergency surgeries for patients who have had an allergic reaction during previous anesthesia, local anesthesia should be used as much as possible, or the use of muscle relaxants and histamine-releasing drugs including morphine and pethidine should be avoided.
Thorough investigation of a patient’s past medical history is important for preventive anaphylaxis. If there is a suspected antigenic substance in the patient's medical history, the best preventive measure is to avoid exposure to that substance and to substances with cross-antigenicity. It makes little sense to use H1 and H2 blockers and corticosteroids as anesthesia premedication. When anaphylaxis occurs, premedication using these drugs suppresses the onset of the initial symptoms and delays the diagnosis of anaphylaxis, which can result in the recognition of anaphylactic shocks only in a severe state. Some experts are of the opinion that such premedication should be avoided [20]. Systematic reviews are generally skeptical about the usefulness of premedication, and there are not enough data to suggest that such premedication is useful for patients with a history of allergic reactions. Therefore, physicians should not rely on the effects of premedication when treating such patients [21].
-
1.
Preoperative diagnosis
Patients corresponding to the following criteria are at high risk of anaphylaxis [1, 3, 4, 22, 23].
-
a.
A definitive diagnosis of allergies caused by drugs administered during past anesthesia.
-
b.
Allergic symptoms during past anesthesia.
-
c.
A previous allergic reaction to latex.
-
a.
-
2.
Preoperative allergy test
Before anesthesia, the risk of allergies should be investigated systematically. All patients do not need to be screened for allergies to anesthetics or products. Patients with atopic dermatitis or a history of allergies to drugs other than anesthetics do not need to be tested for drugs or products used during anesthesia.
For patients with a history of drug allergies, previous anesthesia records should be obtained, and they should be newly tested for muscle relaxants if they are allergic to muscle relaxants. A skin test and IgE test for muscle relaxants and latex are recommended, especially in patients for whom it is not clear which anesthesia method should be used. If it is known, a skin test should be performed for all the drugs listed in the anesthesia records and latex. In the event of an emergency surgery, latex-free surgery should be performed with local anesthesia. If general anesthesia is chosen, muscle relaxants and histamine-releasing drugs should not be used. Skin tests and IgE tests for latex are recommended for patients at risk of latex allergy.
-
3.
Special circumstances and allergy test
-
a.
In principle, nonsteroidal anti-inflammatory drugs (NSAIDs) should not be administered for allergic reactions immediately after the administration of NSAIDs. Cyclooxygenase (COX)-2 inhibitors are often not problematic. Acetaminophen can be administered at reduced doses.
-
b.
Morphine and codeine should not be administered if the patient is allergic to them. Other opioids may be administered.
-
a.
Diagnosis at disease onset (including differential diagnosis)
Summary statements.
-
In the event of vasopressor-resistant anaphylactic shock, treatments for anaphylactic shock should be started even if there is no skin rash.
-
Even if there is no skin rash, anaphylaxis should be suspected if there is a suspect drug involved.
-
Anaphylactic acute coronary syndrome (Kounis syndrome) should be considered as part of the differential diagnosis in the event of an anaphylactic shock.
-
The possibility of the coexistence and differentiation of severe pathological conditions should be considered.
-
Blood for tryptase/histamine testing should be collected at two points, including at the time of onset and at the post-onset reference value.
-
1.
Timing of onset
Approximately 90% of perioperative anaphylaxes develop during anesthesia induction [4], because the administered drugs, including muscle relaxants, antibiotics, intravenous anesthetics, and opioids, and exposure to materials including latex gloves, which cause anaphylaxis, are concentrated during anesthesia induction. Anaphylaxis usually develops within 30 min of exposure, but it may develop within seconds or minutes of exposure and worsen rapidly. In contrast, in some cases, anaphylaxis may develop over hours. Additionally, even if the treatment is effective during the early stages of anesthesia and the causative drug/substance is eliminated, shock may appear several minutes to 10 h later [24]; therefore, the patient should be monitored for 10–20 h after anaphylaxis.
-
2.
Symptoms
Table 1 presents the clinical diagnostic criteria for anaphylaxis. They are generally released in the guidelines of the World Allergy Organization and the Japanese Society of Allergology (Table 1) [25]. Under general anesthesia, the patient cannot complain of subjective symptoms; therefore, gastrointestinal symptoms including abdominal pain and vomiting, and subjective skin symptoms like pruritus and dyspnea cannot be detected. Furthermore, concomitant symptoms including hypotonia, collapse, fainting, and incontinence also remain hidden. If the patient’s consciousness is clear during regional anesthesia, some patients may have complaints of these subjective symptoms.
Table 1 Clinical criteria for diagnosing anaphylaxis Table 2 represents clinical symptoms that can be used to diagnose anaphylaxis during anesthesia. Anaphylaxis during anesthesia is often characterized by a rapid drop in blood pressure and non-response to vasopressors commonly used during anesthesia, including ephedrine and phenylephrine. Although this may be accompanied by skin symptoms, it is overlooked in many cases as an initial symptom, because the patient’s body is usually covered with surgical drapes during surgery. It is not unusual to find skin symptoms only after taking off surgical drapes when anaphylaxis is suspected in cases of vasopressor drug-resistant circulatory collapse. Additionally, since the upper respiratory tract is secured during tracheal intubation, suffocation does not occur due to pharyngeal/laryngeal edema, but bronchial spasms may cause airway stenosis. While differentiating this from an asthma attack is important, mistaking bronchial spasm for tube trouble can delay the appropriate treatment, and respiratory disorders may become more serious, especially in children; therefore, rapid and stringent differential diagnosis is necessary.
Table 2 Clinical symptoms for diagnosing anaphylaxis during anesthesia Approximately 50% of cases of adult perioperative anaphylaxis are noticed through early symptoms including decreased blood pressure, circulatory collapse, and cardiac arrest [22]. Anaphylaxis in children is often noticed via respiratory symptoms including pharyngeal/laryngeal edema and bronchial spasms, and there is generally no large decrease in blood pressure or circulatory collapse [26]. A differential diagnosis for bronchial spasm is a bronchial asthma attack. However, a history of asthma is a risk factor for the onset and severity of anaphylaxis, and an asthma attack may be accompanied by anaphylaxis (Table 2) [22, 26,27,28].
The Ring and Messmer classification is the most commonly used classification for the severity of anaphylaxis: Grade I refers to mucocutaneous symptoms only, Grade II refers to moderate multivisceral signs, Grade III refers to life-threatening mono- or multivisceral signs, and Grade IV refers to cardiac arrest [29]. If circulatory collapse or a bronchial spasm of unknown cause occurs during surgery, surgical drapes must be uncovered to check for skin symptoms and promptly distinguish it from serious situations and symptoms including ventilation problems and heart disease. Mostly, anaphylaxis is accompanied by skin findings including systemic redness due to peripheral vasodilation and extensive urticaria, and mucosal findings including angioedema of the eyelids and lips. However, when vasoconstriction occurs during circulatory collapse due to the stimulation of sympathetic nerves, it may be preceded by symptoms including piloerection, erect nipples, and pallor.
-
3.
Identification of the causative substance
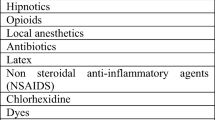
To clinically diagnose anaphylaxis, identifying characteristic symptoms and allergens that are likely to be triggers is important. The most common causative drugs/substances of perioperative anaphylaxis are muscle relaxants, antibacterial agents, latex, and chlorhexidine. Less frequent causes include intravenous anesthetics, opioids, and colloid fluids used during the induction of anesthesia. Additionally, rarer causes include contrast agents, blood transfusion products, preservatives in drug solutions, nonsteroidal anti-inflammatory drugs, povidone in povidone iodine, perfusate, local anesthetics, heparin and other anticoagulants, protamine, and colorants [30].
Antibiotics, muscle relaxants, and intravenous anesthetics often cause anaphylaxis within 30 min of anesthesia induction. Latex, colloid fluids, blood transfusions, protamine, and colorants often cause anaphylaxis 30 min or more after anesthesia induction.
-
4.
Risk factors
Along with the identification of allergens, it is important to confirm the risk factors for anaphylaxis in the patient in advance.
Risk factors for anaphylaxis during anesthesia include female sex, history of anaphylaxis, history of drug allergies, asthma, history of frequent surgery, and diseases caused by mast cell abnormalities [19]. Many patients with anaphylaxis due to muscle relaxants and intravenous anesthetics are women. Risk factors for the aggravation of anaphylaxis include: old age; asthma or hypertension; use of oral antihypertensive drugs including angiotensin converting enzyme inhibitors, angiotensin II antagonists, β-blockers, and calcium channel blockers; high basal tryptase levels; and cardiovascular diseases [5].
-
5.
Laboratory findings
To promptly treat anaphylaxis, diagnosis upon disease onset is made based on the clinical diagnostic criteria. It is useful to capture the increase in plasma tryptase and histamine levels during the early stages of disease onset to aid the definitive diagnosis. Both must be measured at two time points, namely immediately after the onset and before or after the onset as a reference [31, 32].
The main tests that assist the diagnosis of anaphylaxis are tryptase (plasma/serum) and histamine (plasma). Blood samples must be taken soon after anaphylaxis onset [31, 32]. For tryptase, blood samples are collected 15 min to 3 h from onset, and IgE-mediated anaphylaxis is highly likely if the tryptase level is 141% or higher than the reference value or shows an absolute increase of 15–25 µg/mL. The reference value is the value before the onset or 24 h after the onset. However, tryptase levels also increase in cases of myocardial infarction, trauma, and asphyxiation. Additionally, tryptase levels are often normal in children and in the absence of hypotension. As plasma histamine levels return to normal within 15–30 min after onset, blood samples should be collected within 5–10 min after anaphylaxis onset to examine increased histamine levels by hematological examinations. However, N-methylhistamine, a metabolite of histamine, is excreted in the urine 30–60 min after onset and can be detected for several hours [31,32,33,34,35].
-
6.
Differential diagnosis
Table 3 presents the differential diagnosis for anaphylaxis during anesthesia. Anaphylaxis during anesthesia is often noticed on account of a rapid decrease in blood pressure or a ventilatory disorder, and prompt treatment is required. Moreover, the anaphylaxis during anesthesia must be differentiated quickly from other conditions. Pathological conditions similar to anaphylactic shock include a decrease in blood pressure due to sedative/analgesic drugs and a vagal reflex due to tracheal intubation at the time of anesthesia induction. However, these are usually promptly relieved through the use of pressor drugs including ephedrine, phenylephrine, and atropine. Figure 1 illustrates a flowchart of the differential diagnosis upon onset of the reaction. If the patient does not respond to these treatments and shows skin symptoms in addition to other symptoms, treatment for anaphylaxis should be started. At that time, cardiogenic shock and pulmonary embolism (PE) should also be considered as part of the differential diagnosis, depending on the preoperative condition and the risk of deep venous thromboembolism (Fig. 1).
Table 3 Differential diagnosis When sudden ventilatory impairment occurs immediately after anesthesia induction, it may be a result of pharyngeal/laryngeal edema or a bronchial spasm caused by the drug(s). If skin symptoms are observed at this time, the diagnostic criteria for anaphylaxis will be met, and treatments should be started accordingly. If the onset occurs before tracheal intubation, the airway should be secured according to the difficult airway management algorithm. Anaphylaxis may be diagnosed if no skin findings precede the causative drug. However, bronchial spasms due to an asthma attack should also be considered as a differential diagnosis. In all situations, adrenaline administration is the primary treatment. If the patient is suspected to have bronchial spasms by detecting wheezing concomitant with ventilatory impairment while being intubated, he/she may have anaphylaxis even if no skin symptoms are observed, but this must be differentiated from problems caused by tube obstruction or deviation. Particular attention should be paid to newborns and infants (Fig. 1).
If there is a sudden drop in blood pressure during anesthesia, hemorrhagic shock, hypovolemic shock, cardiogenic shock due to heart disease/arrhythmia, PE, cardiac tamponade, and tension pneumothorax must be differentiated. If the patient is resistant to vasopressor drugs and skin symptoms are observed, treatments for anaphylaxis should commence immediately. Even if there are no skin findings, if the condition is subsequently caused by administration of a causative drug, exposure to latex, or blood transfusion, it should immediately be treated as anaphylaxis. At the same time, serious pathological conditions must be differentiated. It is important to check the patient’s hemorrhage status and changes in electrocardiogram, listen to wheezing by auscultation, and in some cases, perform transthoracic wall/esophageal echocardiography and chest X-ray. The above-mentioned pathological conditions and anaphylaxis may coexist, and in such cases, treatment will be diverse. In either case, if affordable, blood samples should be collected for tryptase and histamine tests at two-time points: immediately after onset and 24 h after onset, as a reference value.
Treatment
Summary statements
-
In cases of anaphylaxis, rapid diagnosis and treatment are key to saving a patient’s life.
-
Anaphylactic shocks require treatments similar to cardiopulmonary resuscitation.
-
The first-line treatment for anaphylaxis is adrenaline.
-
Rescue doses of adrenaline may be administered as needed. If repeat doses are necessary, the patient should be started on a continuous intravenous infusion. In case of low blood pressure, intravenous administration of 0.2 µg/kg is required. In case of circulatory collapse, intravenous administration of 0.05–0.3 mg is required. If a venous route cannot be secured, 0.3 mg of adrenaline must be administered by intramuscular injection (0.01 mg/kg for children).
-
The second-line treatments for anaphylaxis are corticosteroid preparations and antihistamines.
-
Vasopressin must be considered for catecholamine-resistant anaphylactic shocks.
-
Administration of vasopressin for catecholamine-resistant anaphylactic shocks must be considered.
-
1.
General rules for treatment.
-
a.
Promptly recognize, diagnose, and treat.
-
b.
Raise the patient’s legs from the supine position when the diagnosis is confirmed.
-
c.
Perform treatments similar to cardiopulmonary resuscitation for circulatory collapse and severe bronchospasm (managements of airway, breathing, and circulation).
-
d.
In critically ill patients, immediately administer 0.2 μg/kg of adrenaline intravenously.
-
e.
Use corticosteroids and antihistamines as the second-line treatments.
-
f.
Administer high flow oxygen.
-
g.
Secure a venous route.
-
h.
Start fluid resuscitation.
-
i.
Perform blood sampling, urine sampling, and β-tryptase measurements for diagnosis.
-
a.
-
2.
Treatment of anaphylactic shock in the operating room
Treatment of anaphylaxis that occurs during anesthesia is not particularly different in Japan from those generally indicated in the guidelines for other countries. Anaphylactic shock is a condition in which, in addition to the anaphylactic reaction, blood pressure drops rapidly and causes altered consciousness, which is a life-threatening condition. A decision should be made with the surgical team about continuation of the surgical procedure, based on the progress of the surgery and severity of the patient’s condition. The patient’s arterial oxygen saturation should be monitored under 100% oxygen ventilation to maximize the prevention of hypoxemia. The patient’s hemodynamics should be closely monitored during intravenous administration of adrenaline [1]. Laryngeal, pharyngeal, and tongue edemas occur frequently in fatal cases of severe anaphylactic shock [36]. In patients who survive severe anaphylactic shock, edema of the lips, face, and extremities are common, but in patients who do not, edema of the larynx and pharynx and edema of the tongue are more frequent [36]. Close attention must be paid to extubation during the early postoperative period and to determine the timing of extubation after confirming the degree of laryngeal/pharyngeal edema. If laryngeal/pharyngeal edema is detected, aggressive securing of the airway and artificial ventilation are performed, after which extubation is performed upon confirming the improvement of the edema.
-
a.
Discontinue the use of all possible causative drugs.
-
b.
Gather help to record the clinical course and treatment details of the patient.
-
c.
Raise the patient’s legs from the supine position.
-
d.
Administer oxygen via a mask (6–8 L/min) or 100% oxygen if the patient is intubated.
-
e.
Secure a venous route if it has not been done.
-
f.
Administer adrenaline.
Administer rescue doses as necessary. Start continuous intravenous infusion if repeat doses are needed (low pressure: administer 0.2 µg/kg intravenously; circulatory collapse: administer 0.05–0.3 mg intravenously).
If a venous route is not available, administer 0.3 mg as an intramuscular injection (0.01 mg/kg for children).
-
g.
Perform tracheal intubation if laryngeal/pharyngeal edema progresses.
-
h.
Ensure crystalloid fluid replenishment (until recovery of blood pressure).
-
1.
5–10 mg/kg during the first 5 min
-
2.
30 mL/kg for children during the first hour
-
3.
Post-resuscitation treatment
-
(1)
Bronchodilators.
Inhalation of β2-agonists is used for ventilation difficulties due to bronchial spasms.
-
(2)
Corticosteroid preparations.
Corticosteroid preparations are used in the second stage of treatment. They may be able to suppress the prolongation of the anaphylactic reaction. There is no evidence for an optimal dose. They should be administered gradually to avoid hypotension.
Anaphylaxis is rarely reported with hydrocortisone use [36, 37].
Adult: Hydrocortisone 200 mg.
Children 12 years or older: Hydrocortisone 200 mg.
-
6–12 years old: Hydrocortisone 100 mg.
-
6 months to 6 years old: Hydrocortisone 50 mg
-
< 6 months old: Hydrocortisone 25 mg.
-
-
(3)
Antihistamines.
Antihistamines are used in the second stage of treatment. Antihistamines alone cannot save a patient’s life. Although evidence for treatment is weak, there are pharmacological applications [38]. H1 blockers reduce histamine-mediated vasodilation and bronchoconstriction. Antihistamines are very safe, although reactions caused by other mediators cannot be suppressed. There is little evidence to support the routine co-administration of H2 blockers.
-
(4)
Cardiovascular agonists.
A recent guideline suggested to use of noradrenaline, vasopressin, and glucagon for shocks that are resistant to adrenaline and fluid loading [13].
-
1.
-
3.
Allergic acute coronary syndrome
Kounis syndrome, first reported by Kounis and Zavras in 1991 [39], is a condition in which an allergic reaction caused by the activation of mast cells and acute coronary syndrome occur simultaneously. Among the various mediators released from mast cells, histamine has a coronary artery spasm effect, and tryptase and chymase destabilize coronary artery plaques. The more severe the allergic reaction, the higher the risk of developing acute coronary syndrome, and the higher the mortality rate when it develops. Therefore, it is important to respond promptly to anaphylaxis. Treatment for anaphylaxis and acute coronary syndrome should be performed at the same time; however, adrenaline should be administered carefully as it tends to exacerbate myocardial ischemia and coronary artery spasm [40]. Morphine is not used, because it stimulates the release of chemical mediators from mast cells.
-
a.
Postoperative diagnosis
Summary statements
-
When anaphylaxis occurs, the anesthesiologist should take the initiative to carry out tests to identify the causative agent.
-
Four to six weeks after the onset of anaphylaxis, a skin test, which is the gold standard for postoperative diagnosis, should be performed.
-
If the prick test is negative, the intradermal test should be performed.
-
Patch tests and drug-induced lymphocyte stimulation tests are not suitable diagnostic tools for anaphylaxis.
-
In vitro tests including the BAT, histamine release test, and allergen-specific IgE measurement should be performed to improve the accuracy of the postoperative diagnosis.
Whenever a patient is diagnosed with anaphylaxis, the physician should determine the causative agent, educate the patient on the dangers of the substance (drug) in writing, and instruct the patient to avoid the antigen. It is essential to diagnose whether the reaction is an immunological or a non-immunological anaphylaxis when the patient’s condition has stabilized after treatment, based on the clinical diagnosis of the anaphylaxis. Confirming the mechanism of the reaction is important for the future prognosis of the patient. If it is an IgE-mediated anaphylaxis, the antigenic substance must be eliminated from the patient's daily life. With drug-induced anaphylaxis, it is critical to determine the drug that has become the antigen. It is important for the physician and patient to know whether the drug can be used in future medical treatment. With immunological anaphylaxis, re-exposure to the antigenic substance is likely to cause more violent reactions than the first exposure. In contrast, with non-immunological anaphylaxis, re-exposure to the causative agent (drug) does not always cause anaphylaxis. If the patient has experienced immunological anaphylaxis, they can be treated appropriately even when anaphylaxis occurs again by always being in possession of a document stating that the causative agent might trigger an anaphylaxis. During early tests, blood samples should be collected to determine the cause of the adverse reaction. A definitive test must be carried out for the causative agent after the antibody level has fully recovered 4–6 weeks after the onset of the adverse reaction. Treatment of anaphylaxis can be considered to be completed upon confirmation of the causative substance along with completion of the treatment of the acute phase of the anaphylaxis shock.
Postoperative diagnosis should be performed to identify the causative agent, as surgery may be postponed due to anaphylaxis, and even if surgery can be performed, the patient may be anesthetized in the future. Currently, the skin test is the gold standard, but it is not 100% accurate. If possible, both in vivo and in vitro tests should be performed to improve the accuracy of the diagnosis. Incorrect identification of the causative agent increases the risk that the patient will develop anaphylaxis again. Furthermore, this can lead to the avoidance of medications that the anesthesiologist would otherwise not need to avoid, which can limit the anesthesiologist's options.
-
1.
In vivo testing.
-
(1)
Skin test.
An in vivo test is recommended as a definitive diagnostic tool for anaphylaxis in all foreign guidelines [1, 3, 4, 23]. A French study reported that 72.9% of cases of severe drug-induced anaphylaxis could be confirmed by skin tests [41]. In addition to consultation with other departments for skin tests, anesthesiologists should consider conducting skin tests themselves, because the test can be performed in consideration of future anesthesia management, and the anesthesiologist is capable of handling any recurrence of anaphylaxis that may occur during the test. Additionally, it is dangerous to narrow down the suspect drugs based on the timing of drug administration and the occurrence of anaphylaxis. As a general rule, all drugs administered before the onset of anaphylaxis should be included in the list of suspect drugs. Histamine dihydrochloride solution (Torii Pharmaceutical Co., Ltd., 10 mg/mL) and physiological saline are used as positive and negative controls, respectively. As antihistamines, antidepressants, and corticosteroids can affect the results of skin tests, their use should be discontinued a few days before the test, if possible [4]. Skin tests are often ineffective against NSAIDs, dextrans, and contrast agents [4] and should be performed 4–6 weeks after the onset of anaphylaxis [1]. The test can be performed before this period, but the results are likely to be false negatives and can only be used as a basis for subsequent treatment decisions if a positive result is obtained [1]. Skin tests may be performed to look for alternative treatments. However, even if there is a negative result on drugs unused in previous anesthesia for the patient, the drug may not necessarily be safe. In other words, when using a negative result as the basis for judgment, it is better to limit the scope to drugs that have previously been administered to the patient. Particular attention should be paid when the causative agent is found to be a muscle relaxant. Although there are only three muscle relaxants available in Japan (rocuronium, vecuronium, and suxamethonium), antigen cross-reactivity has been pointed out for these three drugs. In fact, 40% of patients with rocuronium-induced anaphylaxis have cross-antigen responses to vecuronium, and 44% have cross-antigen responses to suxamethonium [42]. It is also necessary to pay attention to the maximum concentration of the drug used for the skin test (Table 4). This is because if the drug concentration is high, false positives may occur even in healthy individuals who have no history of allergies.
Table 4 Maximum concentration of drug used for skin test -
(a)
Prick test
Prick tests usually use the volar side of the forearm [4]. Since using alcohol-infused cotton may cause nonspecific redness on the skin, the target area should be wiped with normal saline instead. After the suspect drug is dripped onto the skin, a small hole is made in the same area using a lancet needle (bifurcated needle, Tokyo M.I. Company, Inc.). The assessment is performed 15–20 min later [4]. A result is considered positive if the wheal is at least 3 mm larger than the negative control or at least half the size of the positive control [1]. As a general rule, the starting concentration of the suspect drug should be a 1000-fold dilution of the maximum concentration. If the result is negative even after 20 min of waiting, the same test should be repeated using a test solution that is ten times more concentrated [1].
-
(b)
Intradermal test.
An intradermal test is performed when the prick test is negative. The intradermal test uses skin on the volar side of the forearm or the back [1, 3]. Between 0.02 and 0.05 mL (0.03 mL according to The British Society of Allergy and Clinical Immunology: BSACI Guidelines [23]) of the suspect drug is injected into the skin [1, 3]. As a positive control, a histamine dihydrochloride solution (10 μg/mL) at 1:1000 dilution is used. The positive judgment varies according to the guidelines of each country. According to French guidelines, a positive judgment is made if 20 min after drug injection, the wheal is of the same size or twice as large as it was immediately after the drug injection [1]. In contrast, in the British guidelines, a positive judgment is made when the size of the wheal is 3 mm or greater immediately after drug injection and the wheal is accompanied by erythema [23].
-
(a)
-
(2)
Challenge test
The challenge test is used to monitor allergic reactions by administering small amounts at a time of a drug suspected of allergy [7]. There is a high risk of complications including the reproduction of anaphylaxis. This test may be conducted if the suspected drug is a local anesthetic, antibacterial drug, latex, or NSAID, and the skin test is negative. As the usefulness of the challenge test for perioperative anaphylaxis is limited, it should be conducted when the benefits outweigh the risks [1]. For some antibiotics and NSAIDs, metabolites can be allergens; this makes the challenge test the only promising test. The test should be performed at least 1 month after the onset of anaphylaxis. As a general rule, the route of drug administration (intravenous, transdermal, or oral) used should be the same as that used at the onset of anaphylaxis [1].
-
(3)
Patch test
This test examines skin reactions by applying a substance suspected of being an allergen to the arm or back. The patch test is a test for contact dermatitis and drug eruption, and is not applicable when anaphylaxis during anesthesia is suspected [3].
-
(1)
-
2.
In vitro testing
-
(1)
BAT
The BAT identifies allergens by adding an antigen that appears to be an allergen to the peripheral blood samples of patients, and analyzes the activation of basophils with a flow cytometer. CD63 and CD203c are typical markers for activated basophils. For example, in measurements using the Allergenicity Kit® (Beckman Coulter), basophils can be identified from blood cells by combining CD3, CRTH2, and CD203c, and the expression level of CD203c is then measured. CD63 was the mainstream marker, but recently, reports using CD203c have increased [43]. The superiority or inferiority of a marker depends on the drug (See Table 5 [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]). The blood sample used for the test should be fresh, collected within 4 h before the test [66].
Table 5 The diagnostic accuracy of the basophil activation test -
(2)
Histamine release test (HRT).
In the HRT, the amount of histamine released from basophils by antigen stimulation is measured by enzyme-linked immunosorbent assay (ELISA). While HRT has been used for many years, it has not been widely used due to the large amount of sample required and the need to process the blood sample quickly after collection. Today, basophils can be extracted from whole blood using monoclonal antibodies and are then made to react with antigens, making testing possible only with a small amount of blood. In recent years, a method for quantifying the amount of histamine released from basophils using a flow cytometer has also been developed [67].
-
(3)
Measurement of tryptase and histamine
Although measuring tryptase and histamine levels is helpful for the definitive diagnosis of anaphylaxis, it should be performed immediately after the onset of anaphylaxis and is omitted in this chapter.
-
(4)
Measurement of allergen-specific IgE
In cases where IgE is involved in the development of anaphylaxis, IgE specific for the causative agent (allergen) of anaphylaxis should be detectable in the patient's blood. To detect the specific IgE, the drug must be isolated and reacted with the patient's plasma [68]. The method used to measure allergen-specific IgE used to be called the radioallergosorbent test method, because it used radioisotopes. However, currently, the mainstream approaches used include a method that identifies IgE by fluorescent substances without the use of radioisotopes (CAP-fluorescence enzyme immunoassay) and the ELISA method in which an antibody that recognizes IgE is labeled with an enzyme and quantified. In Japan, several companies sell reagents for measuring allergen-specific IgE, but most of them are used for testing allergies to food, plants, and animals. The reagents sold by Thermo Fisher Diagnostics Co., Ltd. can measure IgE specific for perioperative drugs including β-lactam antibiotics, suxamethonium, morphine, chlorhexidine, and protamine. Per previous reports, the measurements of specific IgE for β-lactam antibiotics are not highly sensitive or specific, with sensitivity and specificity values of 0–85% and 52–100%, respectively [69]. Therefore, it is not necessary to routinely measure IgE specific to β-lactam antibiotics [1]. In contrast, the measurements of IgE specific for muscle relaxants were reported to have relatively high sensitivity (38.5–92%) and specificity (92–100%) [69]. Measurement of IgE specific for rocuronium involves the use of a kit that was developed for research purposes, and it is currently difficult to measure rocuronium-specific IgE in Japan. The usefulness of the skin test for opioids is unclear, and there are no reports on the measurements of sensitivity and specificity of IgE that is specific for morphine. In contrast, many patients with muscle relaxant-induced anaphylaxis have specific IgE for morphine [70]. In fact, 5–10% of patients are morphine-specific IgE carriers. Therefore, even if specific IgE for morphine can be detected, it cannot be used to definitively diagnose anaphylaxis caused by muscle relaxants and opioids [69]. Measurements of specific IgE for the disinfectant chlorhexidine were reported to demonstrate good sensitivity and specificity, with values of 84.2–91.6% and 93.7–100%, respectively [71, 72].
-
(5)
Drug-induced lymphocyte stimulation test (DLST)
DLST is a test that utilizes the blastogenesis reaction of lymphocytes stimulated by substances, and is applicable for type IV (delayed) allergies. In contrast, most anaphylaxis events are type I allergies, which are primarily caused by the activation of mast cells and basophils. Therefore, performing DLST is of little significance in patients with anaphylaxis. In fact, DLST performed on patients with anaphylaxis to anesthetics and muscle relaxants did not yield good results [73].
-
(1)
-
3.
Use of postoperative diagnosis data
-
Even if the causative agent of anaphylaxis is identified, the information is not particularly meaningful unless it is passed on to the patient and anesthesiologist, and used to manage the next event of anesthesia. The results of the postoperative diagnosis should be recorded in the medical records of the patient and communicated to the patient in writing, taking into consideration the possibility that the patient may be anesthetized at another medical facility in the future [1]. In such a situation, both the non-proprietary name and trade name of the causative substance should be indicated.
-
References
Mertes PM, Malinovsky JM, Jouffroy L, Aberer W, Terreehorst I, Brockow K, Demoly P, Working Group of the SFAR and SF, ENDA, EAACI Interest Group on Drug Allergy. Reducing the risk of anaphylaxis during anesthesia: 2011 updated guidelines for clinical practice. J Investig Allergol Clin Immunol. 2011;21(6):442–53.
Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI, Bernstein JA, Burks AW, Feldweg AM, Fink JN, Greenberger PA. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126(3):477-80 e1-42.
Harper NJ, Dixon T, Dugué P, Edgar DM, Fay A, Gooi HC, Herriot R, Hopkins P, Hunter JM, Mirakian R, Pumphrey RS, Seneviratne SL, Walls AF, Williams P, Wildsmith JA, Wood P, Nasser AS, Powell RK, Mirakhur R, Soar J, Working Party of the Association of Anaesthetists of Great Britain and Ireland. Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64(2):199–211.
Kroigaard M, Garvey LH, Gillberg L, Johansson SG, Mosbech H, Florvaag E, Harboe T, Eriksson LI, Dahlgren G, Seeman-Lodding H, Takala R, Wattwil M, Hirlekar G, Dahlén B, Guttormsen AB. Scandinavian Clinical Practice Guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand. 2007;51(6):655–70.
Simons FE, Ebisawa M, Sanchez-Borges M, Thong BY, Worm M, Tanno LK, Lockey RF, El-Gamal YM, Brown SG, Park HS, Sheikh A. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ J. 2015;8(1):32.
Kolawole H, Marshall SD, Crilly H, Kerridge R, Roessler P. Australian and New Zealand anaesthetic allergy group/Australian and New Zealand college of anaesthetists perioperative anaphylaxis management guidelines. Anaesth Intensive Care. 2017;45(2):151–8.
The Anaphylaxis Countermeasures Special Committee of the Japanese Society of Allergology. Anaphylaxis Guideline. 2014.
Takazawa T, Sabato V, Ebo DG. In vitro diagnostic tests for perioperative hypersensitivity, a narrative review: potential, limitations, and perspectives. Br J Anaesth. 2019;123(1):e117–25.
Mertes PM, Ebo DG, Garcez T, Rose M, Sabato V, Takazawa T, Cooke PJ, Clarke RC, Dewachter P, Garvey LH, Guttormsen AB, Hepner DL, Hopkins PM, Khan DA, Kolawole H, Kopac P, Krøigaard M, Laguna JJ, Marshall SD, Platt PR, Sadleir PHM, Savic LC, Savic S, Volcheck GW, Voltolini S. Comparative epidemiology of suspected perioperative hypersensitivity reactions. Br J Anaesth. 2019;123(1):e16–28.
Mertes PM, Volcheck GW, Garvey LH, Takazawa T, Platt PR, Guttormsen AB, Tacquard C. Epidemiology of perioperative anaphylaxis. Presse Med. 2016;45(9):758–67.
Gibbs NM, Sadleir PH, Clarke RC, Platt PR. Survival from perioperative anaphylaxis in Western Australia 2000–2009. Br J Anaesth. 2013;111(4):589–93.
Savic LC, Kaura V, Yusaf M, Hammond-Jones AM, Jackson R, Howell S, Savic S, Hopkins PM. Incidence of suspected perioperative anaphylaxis: a multicenter snapshot study. J Allergy Clin Immunol Pract. 2015;3(3):454-5 e1.
Garvey LH, Dewachter P, Hepner DL, Mertes PM, Voltolini S, Clarke R, Cooke P, Garcez T, Guttormsen AB, Ebo DG, Hopkins PM, Khan DA, Kopac P, Krøigaard M, Laguna JJ, Marshall S, Platt P, Rose M, Sabato V, Sadleir P, Savic L, Savic S, Scherer K, Takazawa T, Volcheck GW, Kolawole H. Management of suspected immediate perioperative allergic reactions: an international overview and consensus recommendations. Br J Anaesth. 2019;123(1):e50–64.
Garvey LH, Ebo DG, Mertes PM, Dewachter P, Garcez T, Kopac P, Laguna JJ, Chiriac AM, Terreehorst I, Voltolini S, Scherer K. An EAACI position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy. 2019;74(10):1872–84.
Mitsuhata H, Hasegawa J, Matsumoto S, Ogawa R. The epidemiology and clinical features of anaphylactic and anaphylactoid reactions in the perioperative period in Japan: a survey with a questionnaire of 529 hospitals approved by Japan Society of Anesthesiology. Masui. 1992;41(11):1825–31.
Horiuchi T, Takazawa T, Orihara M, Sakamoto S, Nagumo K, Saito S. Drug-induced anaphylaxis during general anesthesia in 14 tertiary hospitals in Japan: a retrospective, multicenter, observational study. J Anesth. 2021;35(1):154–60.
Harper NJN, Cook TM, Garcez T, Farmer L, Floss K, Marinho S, Torevell H, Warner A, Ferguson K, Hitchman J, Egner W, Kemp H, Thomas M, Lucas DN, Nasser S, Karanam S, Kong KL, Farooque S, Bellamy M, McGuire N. Anaesthesia, surgery, and life-threatening allergic reactions: epidemiology and clinical features of perioperative anaphylaxis in the 6th National Audit Project (NAP6). Br J Anaesth. 2018;121(1):159–71.
Laxenaire MC. Epidemiology of anesthetic anaphylactoid reactions. Fourth multicenter survey (July 1994-December 1996). Ann Fr Anesth Reanim. 1999;18(7):796–809.
Reitter M, Petitpain N, Latarche C, Cottin J, Massy N, Demoly P, Gillet P, Mertes PM. Fatal anaphylaxis with neuromuscular blocking agents: a risk factor and management analysis. Allergy. 2014;69(7):954–9.
Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97(5):1381–95.
Tramer MR, von Elm E, Loubeyre P, Hauser C. Pharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic review. BMJ. 2006;333(7570):675.
Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, Brown SG, Camargo CA, Cydulka R, Galli SJ, Gidudu J, Gruchalla RS, Harlor AD, Hepner DL, Lewis LM, Lieberman PL, Metcalfe DD, O’Connor R, Muraro A, Rudman A, Schmitt C, Scherrer D, Simons FE, Thomas S, Wood JP, Decker WW. Second symposium on the definition and management of anaphylaxis: summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann Emerg Med. 2006;47(4):373–80.
Ewan PW, Dugué P, Mirakian R, Dixon TA, Harper JN, Nasser SM, BSACI. BSACI guidelines for the investigation of suspected anaphylaxis during general anaesthesia. Clin Exp Allergy. 2010;40(1):15–31.
Ellis AK, Day JH. Incidence and characteristics of biphasic anaphylaxis: a prospective evaluation of 103 patients. Ann Allergy Asthma Immunol. 2007;98(1):64–9.
Sampson HA, Muñoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, Decker WW, Furlong TJ, Galli SJ, Golden DB, Gruchalla RS, Harlor AD, Hepner DL, Howarth M, Kaplan AP, Levy JH, Lewis LM, Lieberman PL, Metcalfe DD, Murphy R, Pollart SM, Pumphrey RS, Rosenwasser LJ, Simons FE, Wood JP, Camargo CA. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005;115(3):584–91.
Muraro A, Roberts G, Clark A, Eigenmann PA, Halken S, Lack G, Moneret-Vautrin A, Niggemann B, Rancé F, EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007;62(8):857–71.
Mertes PM, Alla F, Tréchot P, Auroy Y, Jougla E. Anaphylaxis during anesthesia in France: an 8-year national survey. J Allergy Clin Immunol. 2011;128(2):366–73.
Webb LM, Lieberman P. Anaphylaxis: a review of 601 cases. Ann Allergy Asthma Immunol. 2006;97(1):39–43.
Dewachter P, Mouton-Faivre C, Emala CW. Anaphylaxis and anesthesia: controversies and new insights. Anesthesiology. 2009;111(5):1141–50.
Mali S. Anaphylaxis during the perioperative period. Anesth Essays Res. 2012;6(2):124–33.
Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83(5):1551–5.
van der Linden PW, Hack CE, Poortman J, Vivie-Kipp YC, Struyvenberg A, van der Zwan JK. Insect-sting challenge in 138 patients: relation between clinical severity of anaphylaxis and mast cell activation. J Allergy Clin Immunol. 1992;90(1):110–8.
Halmerbauer G, Hauk P, Forster J, Urbanek R, Kaufmehl K, Koller DY. In vivo histamine release during the first minutes after deliberate sting challenges correlates with the severity of allergic symptoms. Pediatr Allergy Immunol. 1999;10(1):53–7.
Smith PL, Kagey-Sobotka A, Bleecker ER, Traystman R, Kaplan AP, Gralnick H, Valentine MD, Permutt S, Lichtenstein LM. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980;66(5):1072–80.
van der Linden PW, Struyvenberg A, Kraaijenhagen RJ, Hack CE, van der Zwan JK. Anaphylactic shock after insect-sting challenge in 138 persons with a previous insect-sting reaction. Ann Intern Med. 1993;118(3):161–8.
Greenberger PA. Anaphylactic and anaphylactoid causes of angioedema. Immunol Allergy Clin N Am. 2006;26(4):753–67.
Corominas N, Mane JM, Codina C, Paz MA, Ribas J. Hydrocortisone anaphylaxis: a new case report. Pharm Weekbl Sci. 1992;14(3):93–4.
Lin RY, Curry A, Pesola GR, Knight RJ, Lee HS, Bakalchuk L, Tenenbaum C, Westfal RE. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000;36(5):462–8.
Kounis NG, Zavras GM. Histamine-induced coronary artery spasm: the concept of allergic angina. Br J Clin Pract. 1991;45(2):121–8.
Kounis NG. Kounis syndrome: an update on epidemiology, pathogenesis, diagnosis and therapeutic management. Clin Chem Lab Med. 2016;54(10):1545–59.
Renaudin JM, Beaudouin E, Ponvert C, Demoly P, Moneret-Vautrin DA. Severe drug-induced anaphylaxis: analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy. 2013;68(7):929–37.
Sadleir PH, Clarke RC, Bunning DL, Platt PR. Anaphylaxis to neuromuscular blocking drugs: incidence and cross-reactivity in Western Australia from 2002 to 2011. Br J Anaesth. 2013;110(6):981–7.
Uyttebroek AP, Sabato V, Faber MA, Cop N, Bridts CH, Lapeere H, De Clerck LS, Ebo DG. Basophil activation tests: time for a reconsideration. Expert Rev Clin Immunol. 2014;10(10):1325–35.
Abuaf N, Rajoely B, Ghazouani E, Levy DA, Pecquet C, Chabane H, Leynadier F. Validation of a flow cytometric assay detecting in vitro basophil activation for the diagnosis of muscle relaxant allergy. J Allergy Clin Immunol. 1999;104(2 Pt 1):411–8.
Abuaf N, Rostane H, Rajoely B, Gaouar H, Autegarden JE, Leynadier F, Girot R. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin Exp Allergy. 2008;38(6):921–8.
De Week AL, Sanz ML, Gamboa PM, Aberer W, Sturm G, Bilo MB, Montroni M, Blanca M, Torres MJ, Mayorga L, Campi P, Manfredi M, Drouet M, Sainte-Laudy J, Romano A, Merk H, Weber JM, Jermann TM. Diagnosis of immediate-type beta-lactam allergy in vitro by flow-cytometric basophil activation test and sulfidoleukotriene production: a multicenter study. J Investig Allergol Clin Immunol. 2009;19(2):91–109.
Eberlein B, Leon Suarez I, Darsow U, Rueff F, Behrendt H, Ring J. A new basophil activation test using CD63 and CCR3 in allergy to antibiotics. Clin Exp Allergy. 2010;40(3):411–8.
Ebo DG, Bridts CH, Hagendorens MM, Mertens CH, De Clerck LS, Stevens WJ. Flow-assisted diagnostic management of anaphylaxis from rocuronium bromide. Allergy. 2006;61(8):935–9.
Gamboa PM, Garcia-Aviles MC, Urrutia I, Antepara I, Esparza R, Sanz ML. Basophil activation and sulfidoleukotriene production in patients with immediate allergy to betalactam antibiotics and negative skin tests. J Investig Allergol Clin Immunol. 2004;14(4):278–83.
Garcia-Ortega P, Marin A. Usefulness of the basophil activation test (BAT) in the diagnosis of life-threatening drug anaphylaxis. Allergy. 2010;65(9):1204.
Hagau N, Gherman-Ionica N, Sfichi M, Petrisor C. Threshold for basophil activation test positivity in neuromuscular blocking agents hypersensitivity reactions. Allergy Asthma Clin Immunol. 2013;9(1):42.
Horiuchi T, Takazawa T, Orihara M, Sakamoto S, Yokohama A, Takahashi J, Tomioka A, Yoshida N, Hagiwara K, Saito S. Required cefazolin concentration to maximize diagnostic accuracy of the basophil activation test for cefazolin-induced anaphylaxis. J Anesth. 2018;32(6):797–805.
Horiuchi T, Yokohama A, Orihara M, Tomita Y, Tomioka A, Yoshida N, Takahashi K, Saito S, Takazawa T. Usefulness of basophil activation tests for diagnosis of sugammadex-induced anaphylaxis. Anesth Analg. 2018;126(5):1509–16.
Kvedariene V, Kamey S, Ryckwaert Y, Rongier M, Bousquet J, Demoly P, Arnoux B. Diagnosis of neuromuscular blocking agent hypersensitivity reactions using cytofluorimetric analysis of basophils. Allergy. 2006;61(3):311–5.
Leysen J, Bridts CH, De Clerck LS, Vercauteren M, Lambert J, Weyler JJ, Stevens WJ, Ebo DG. Allergy to rocuronium: from clinical suspicion to correct diagnosis. Allergy. 2011;66(8):1014–9.
Monneret G, Benoit Y, Debard AL, Gutowski MC, Topenot I, Bienvenu J. Monitoring of basophil activation using CD63 and CCR3 in allergy to muscle relaxant drugs. Clin Immunol. 2002;102(2):192–9.
Pinnobphun P, Buranapraditkun S, Kampitak T, Hirankarn N, Klaewsongkram J. The diagnostic value of basophil activation test in patients with an immediate hypersensitivity reaction to radiocontrast media. Ann Allergy Asthma Immunol. 2011;106(5):387–93.
Salas M, Gomez F, Fernandez TD, Doña I, Aranda A, Ariza A, Blanca-López N, Mayorga C, Blanca M, Torres MJ. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy. 2013;68(9):1203–6.
Sanz ML, Gamboa PM, Antépara I, Uasuf C, Vila L, Garcia-Avilés C, Chazot M, De Weck AL. Flow cytometric basophil activation test by detection of CD63 expression in patients with immediate-type reactions to betalactam antibiotics. Clin Exp Allergy. 2002;32(2):277–86.
Sudheer PS, Hall JE, Read GF, Rowbottom AW, Williams PE. Flow cytometric investigation of peri-anaesthetic anaphylaxis using CD63 and CD203c. Anaesthesia. 2005;60(3):251–6.
Torres MJ, Ariza A, Fernández J, Moreno E, Laguna JJ, Montañez MI, Ruiz-Sanchez AJ, Blanca M. Role of minor determinants of amoxicillin in the diagnosis of immediate allergic reactions to amoxicillin. Allergy. 2010;65(5):590–6.
Torres MJ, Padial A, Mayorga C, Fernández T, Sanchez-Sabate E, Cornejo-García JA, Antúnez C, Blanca M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin Exp Allergy. 2004;34(11):1768–75.
Torres MJ, Romano A, Blanca-Lopez N, Doña I, Canto G, Ariza A, Aranda A, Montañez MI, Mayorga C, Blanca M. Immunoglobulin E-mediated hypersensitivity to amoxicillin: in vivo and in vitro comparative studies between an injectable therapeutic compound and a new commercial compound. Clin Exp Allergy. 2011;41(11):1595–601.
Trcka J, Schmidt C, Seitz CS, Brocker EB, Gross GE, Trautmann A. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? AJR Am J Roentgenol. 2008;190(3):666–70.
Uyttebroek AP, Sabato V, Leysen J, Bridts CH, De Clerck LS, Ebo DG. Flowcytometric diagnosis of atracurium-induced anaphylaxis. Allergy. 2014;69(10):1324–32.
Sturm GJ, Kranzelbinder B, Sturm EM, Heinemann A, Groselj-Strele A, Aberer W. The basophil activation test in the diagnosis of allergy: technical issues and critical factors. Allergy. 2009;64(9):1319–26.
Ebo DG, Bridts CH, Mertens CH, Hagendorens MM, Stevens WJ, De Clerck LS. Analyzing histamine release by flow cytometry (HistaFlow): a novel instrument to study the degranulation patterns of basophils. J Immunol Methods. 2012;375(1–2):30–8.
Ebo DG, Leysen J, Mayorga C, Rozieres A, Knol EF, Terreehorst I. The in vitro diagnosis of drug allergy: status and perspectives. Allergy. 2011;66(10):1275–86.
Decuyper II, Ebo DG, Uyttebroek AP, Hagendorens MM, Faber MA, Bridts CH, De Clerck LS, Sabato V. Quantification of specific IgE antibodies in immediate drug hypersensitivity: more shortcomings than potentials? Clin Chim Acta. 2016;460:184–9.
Ebo DG, Venemalm L, Bridts CH, Degerbeck F, Hagberg H, De Clerck LS, Stevens WJ. Immunoglobulin E antibodies to rocuronium: a new diagnostic tool. Anesthesiology. 2007;107(2):253–9.
Anderson J, Rose M, Green S, Fernando SL. The utility of specific IgE testing to chlorhexidine in the investigation of perioperative adverse reactions. Ann Allergy Asthma Immunol. 2015;114(5):425–61.
Opstrup MS, Malling HJ, Krøigaard M, Mosbech H, Skov PS, Poulsen LK, Garvey LH. Standardized testing with chlorhexidine in perioperative allergy—a large single-centre evaluation. Allergy. 2014;69(10):1390–6.
Janot C, Moneret-Vautrin DA. Study of allergy to general anesthetics and muscle relaxants using the lymphoblast transformation test. Ann Fr Anesth Reanim. 1983;2(1):39–43.
Funding
The creation of this guide was funded by the Japanese Society of Anesthesiologists (JSA). This guide will be published and accessible free of charge online via the JSA’s official web site.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer
This guide is designed to provide anesthesiologists with useful information for the prevention, early diagnosis and treatment, and postoperative diagnosis of anaphylaxis during anesthesia. Many recommendations contained in this guide are based on expert opinion and case reports in the absence of high-quality evidence, because anaphylaxis is a relatively rare condition. This document aims to assist anesthesiologists’ decision making in clinical settings, and these guidelines are not recommended to general physicians.
Practical guidelines for the response to perioperative anaphylaxis were published in Japanese in February 2021. We share these guidelines in English with healthcare professionals and the general public around the world to improve medical practice and patient outcomes.
Objective
This practical guideline is not intended for all cases of anaphylaxis, but only for those that occur during anesthesia. The major differences between anesthetized patients and general patients are the differences in the causes of anaphylaxis and the availability of venous routes and airways at the onset of anaphylaxis. Additionally, since patients tend to be fully monitored, it is easy to notice abnormal vital signs, but it can be difficult to notice the emergence of skin symptoms in areas where the skin is covered by surgical drapes.
Target audience
The target audience of this guide is anesthesiologists who conduct anesthetic management in operating rooms. This guide is not recommended to general physicians, because anaphylaxis during anesthesia is a special condition in which patients are anesthetized, and most cases are already maintained with an airway, intubated, and maintained under continuous monitoring of vital signs. Furthermore, anesthesiologists are specialists in resuscitation and are familiar with the use of adrenaline and defibrillators that are present in the operating rooms.
About this article
Cite this article
Takazawa, T., Yamaura, K., Hara, T. et al. Practical guidelines for the response to perioperative anaphylaxis. J Anesth 35, 778–793 (2021). https://doi.org/10.1007/s00540-021-03005-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-021-03005-8