Abstract
Colorectal cancer is a major cause of death in Japan, where it accounts for the largest number of deaths from malignant neoplasms among women and the third largest number among men. Many new methods of treatment have been developed during recent decades. The Japanese Society for Cancer of the Colon and Rectum Guidelines 2014 for treatment of colorectal cancer (JSCCR Guidelines 2014) have been prepared as standard treatment strategies for colorectal cancer, to eliminate treatment disparities among institutions, to eliminate unnecessary treatment and insufficient treatment, and to deepen mutual understanding among health-care professionals and patients by making these guidelines available to the general public. These guidelines have been prepared as a result of consensuses reached by the JSCCR Guideline Committee on the basis of careful review of evidence retrieved by literature searches and taking into consideration the medical health insurance system and actual clinical practice in Japan. They can, therefore, be used as a guide for treating colorectal cancer in clinical practice. More specifically, they can be used as a guide to obtaining informed consent from patients and choosing the method of treatment for each patient. As a result of the discussions of the Guideline Committee, controversial issues were selected as clinical questions, and recommendations were made. Each recommendation is accompanied by a classification of the evidence and a classification of recommendation categories, on the basis of consensus reached by Guideline Committee members. Here we present the English version of the JSCCR Guidelines 2014.
Similar content being viewed by others
Introduction
1. Guideline objectives
The incidence and mortality of colorectal cancer have substantially increased in Japan recently. According to vital statistics for Japan in 2012, colorectal cancer accounted for the largest number of deaths from malignant neoplasms among women and the third largest number among men, after lung cancer and gastric cancer. The number of deaths from colorectal cancer per unit population has increased approximately tenfold during the past 50 years. Many new treatment methods have been developed during that time, and their use in combination with advances in diagnostic methods has led to a steady improvement in the results of treatment. However, different treatment is used among medical institutions in Japan that provide medical care for patients with colorectal cancer, and the differences may lead to differences in the results of treatment.
In such circumstances, the JSCCR Guidelines 2014 for treatment of colorectal cancer, which are intended for doctors (general practitioners and specialists) who provide medical care for patients with colorectal cancer in different disease stages and conditions, have been prepared for four purposes:
-
1.
to disseminate standard treatment strategies for colorectal cancer;
-
2.
to eliminate disparities among institutions in terms of treatment;
-
3.
to eliminate unnecessary treatment and insufficient treatment; and
-
4.
to deepen mutual understanding among health-care professionals and patients by making these guidelines available to the general public [1].
Achievements expected as a result of these guidelines are:
-
1.
improvement of treatment of colorectal cancer in Japan;
-
2.
improvement of the results of treatment;
-
3.
reduction of human and financial burden; and
-
4.
increased benefits for patients.
2. How to use these guidelines
These guidelines have been as a result of consensuses reached by the Guideline Committee of the Japanese Society for Cancer of the Colon and Rectum, on the basis of careful review of evidence retrieved by literature searches and taking into consideration the medical health insurance system and clinical practice in Japan. They can, therefore, be used as a guide for treating colorectal cancer in clinical practice. More specifically, they can be used as a guide to obtaining informed consent from patients and choosing the method of treatment for each patient. However, these guidelines provide only general recommendations for choosing treatment strategies for colorectal cancer, and they do not control or limit treatment strategies or treatment methods that are not described herein. These guidelines can also be used as a document to explain the rationale for selecting treatment strategies and treatment methods that differ from those described in the guidelines.
The Japanese Society for Cancer of the Colon and Rectum (JSCCR) is responsible for the statements in these guidelines. However, the personnel directly in charge of treatment, not the JSCCR or the Guideline Committee, are responsible for the outcome of treatment.
3. Users
The users of these guidelines are mainly clinical doctors engaged in all aspects of the medical treatment of colorectal cancer.
4. How to develop these guidelines
1) Recording methods
We adopted the concept from the first edition in which the treatment policy algorithm was disclosed and a simple explanation thereof provided, and added further comments with regard to categories requiring additional explanation. Since the 2009 edition, topics of debate have been raised as clinical questions (CQs) and included with recommendations added. In the 2014 edition, this practice was continued, with corrections and additions made to the CQs on the basis of knowledge acquired since the 2010 version.
2) Evidence level and strength of recommendations of CQs
The recommendations added to CQs included the evidence level and the strength of recommendations determined by use of the following guidance.
2-1) Evidence level
Papers relating to the CQs were comprehensively collected and evidence in individual papers relating to critical outcomes included in the CQs was divided into groups by study design [2]. The literature level and the body of evidence (Table 1) were evaluated with reference to the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system [3–25], before determining the final CQ evidence level (Table 2).
2-2) Strength of recommendations
Draft recommendation statements and the strength of the recommendations were based on outcomes and the level of evidence obtained by use of the process described above and were evaluated at a consensus meeting of the Guideline Committee.
The draft recommendations were evaluated on the basis of four categories:
-
①
quality of evidence;
-
②
patients’ views and preferences;
-
③
benefits and harm, and
-
④
cost effectiveness.
The strength of recommendation (Table 3) was determined by vote, on the basis of the Delphi method, with those reaching a consensus of opinion of 70 % or more committee members determined as having been agreed upon. Items not reaching consensus after a single vote were debated once again, with the results of the first vote disclosed and additional information on the situation relating to clinical practice in Japan provided. Discussion and voting was repeated until a consensus was reached. No strength of recommendation was presented in CQs.
5. Literature search
At first, the literature search was performed for the following 12 broad categories. Then, a further search was conducted, as needed, with additional search techniques.
-
(1)
Endoscopic treatment of colorectal cancer
-
(2)
Treatment of Stage 0 to Stage III colorectal cancer [26]
-
(3)
Treatment of Stage IV colorectal cancer [26]
-
(4)
Treatment of liver metastases of colorectal cancer
-
(5)
Treatment of lung metastases of colorectal cancer
-
(6)
Treatment of recurrent colorectal cancer
-
(7)
Adjuvant chemotherapy for colorectal cancer
-
(8)
Chemotherapy for unresectable colorectal cancer
-
(9)
Adjuvant radiotherapy for colorectal cancer
-
(10)
Palliative radiotherapy for colorectal cancer
-
(11)
Palliative care for colorectal cancer
-
(12)
Surveillance after surgery for colorectal cancer.
To survey the latest literature, in addition to the papers used for reference in the previous edition, the PubMed and Ichushi-Web databases were selected for the search, and English and Japanese literature was searched in both databases from January 2008 to March 2012. The task of searching was shared by 4 members of the medical library; the 4 members created a search formula by discussion with the Committee members in charge of each item and collected literature during the search period (March 2012). For categories 7 and 8, however, April 2010 was set as the end of the search period. In addition, secondary documents such as UpToDate and literature collected by manual searching were added and critically examined as needed, and other documents such as minutes and guidelines were included as necessary. In addition to the 8,043 documents extracted in the previous literature search (5,305 PubMed documents and 2,738 Ichushi documents), a further 2,213 documents were selected by use of the study design from the 2,917 documents (2,088 PubMed documents and 829 Ichushi documents) extracted during the literature search for the current edition. and critically examined (Table 4).
6. Funding
Preparation of these guidelines was funded by the JSCCR. No financial support was received from any other organization or corporation.
7. Conflicts of interest
1) The following corporations were disclosed by self-declaration of the Guideline Committee members and Guideline Evaluation Committee members
AstraZeneca K.K., Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Olympus Medical Systems Co., Ltd., Van Medical Co., Ltd., Synergy International, Inc., Tsumura & Co., Yakult Honsha Co., Ltd., Kawasumi Laboratories, Inc., Covidien Japan Co., Ltd., Shionogi & Co., Ltd., Daiichi Sankyo Company, Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Novartis Pharma K.K., Bayer Yakuhin Ltd., Pfizer Japan Inc., Bristol-Myers Squib Company, MerkSerono.
2) Overcoming possible conflicts of interest
The members of the Guideline Committee and the Guideline Evaluation Committee were from a diverse range of disciplines, including surgery, internal medicine, radiology, pathology, etc., to minimize the possibility of biased opinion. Each recommendation was determined not the basis of an individual opinion but on the basis of voting by all the committee members, with consensus prioritized.
Treatment guidelines for colorectal cancer
Chapter 1: Treatment strategies for Stage 0 to Stage III colorectal cancer [26]
-
1.
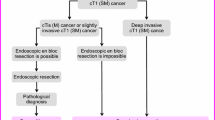
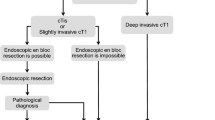
Endoscopic treatment (Fig. 1)
-
General principles underlying indications for endoscopic resection
-
There is little possibility of lymph node metastasis, and the size and location of the tumor make en bloc resection possible.
-
Indication criteria for endoscopic resection:
-
(1)
Intramucosal carcinoma or carcinoma with slight submucosal invasion
-
(2)
Size does not matter
-
(3)
Any macroscopic type
-
(1)
-
Endoscopic treatment is a method of endoscopically resecting lesions in the large bowel and of collecting the resected specimens.
-
Endoscopic treatment methods are polypectomy,note 1 endoscopic mucosal resection (EMR),note 2 and endoscopic submucosal dissection (ESD).note 3
-
In determining the indication for endoscopic treatment and the method of treatment, information on the size, predicted depth of invasion, and morphology of the tumor is essential.
-
Comments
-
①
Endoscopic resection is intended for both diagnosis and treatment. It consists in total excisional biopsy in which curability and the need for additional intestinal resection are assessed by histopathological examination of the resected specimens (CQ-1).
-
②
En bloc resection is desirable for accurate diagnosis of the status of carcinoma invasion in the resection margin and the deepest area.
-
2 cm is the largest size of a tumor that can be easily resected en bloc by polypectomy or snare EMR [27] (CQ-2).
-
Colorectal ESD is an “endoscopic resection technique which enables en-bloc resection of a tumor, irrespective of size”, which was approved for implementation under health insurance in April 2014 with regard to “early-stage malignant tumors”. Given the high likelihood of technically difficult complications (perforations), however, it should only be implemented after sufficient consideration of the level of skill of the endoscopist performing the procedure. Tumors with a diameter between 2 and 5 cm are currently covered by insurance (CQ-3).
-
EMRC (EMR using a cap) is reported to involve a high risk of perforation when used for colon lesions.
-
If the preoperative diagnosis is cancer accompanied by adenoma (intramucosal carcinoma), piecemeal resection of the adenoma can be performed while avoiding division of the cancerous area. It should be noted, however, that piecemeal resection is associated with a high incidence of incomplete resection and high local recurrence [27].
- Note 1:
-
Polypectomy. In this method, a snare is placed on the stalk of the lesion, and the lesion is electrocauterized by use of a high-frequency current. This method is mainly used for protruding lesions.
- Note 2:
-
EMR. In this method, the lesion is elevated by local injection of a liquid, for example physiological saline, into the submucosa, and the lesion is electrocauterized the same as in polypectomy. This method includes the snare method [28] and EMR using a cap (EMRC). It is mainly used for superficial tumors and large sessile lesions.
- Note 3:
-
ESD. In this technique, the lesion is elevated by local injection of a liquid, for example sodium hyaluronate solution, into the submucosa of the perilesional area; circumferential incision of the mucosa surrounding the lesion, dissection of the submucosa with a special knife, and en bloc resection are then performed [28]. ESD is mainly indicated for large tumors, especially for early cancers that cannot be resected by EMR.
-
2.
Surgical treatment (Fig. 2)
-
The extent of lymph node dissection to be performed during colorectal cancer surgery is determined on the basis of the preoperative clinical findings, and on the extent of lymph node metastasis and depth of tumor invasion by the tumor observed intraoperatively.
-
If lymph node metastasis is recognized, or suspected on the basis of the preoperative/intraoperative findings, D3 dissection is performed.
-
If no lymph node metastases are observed on the basis of preoperative and/or intraoperative diagnostic findings, lymph node dissection is performed on the basis of the depth of tumor invasion [29].
-
(1)
Lymph node dissection is unnecessary for pTis (M) cancer (D0), because pTis (M) cancer is not accompanied by lymph node metastasis; however, D1 dissection can be performed because the accuracy of the preoperative diagnosis of invasion depth may be insufficient.
-
(2)
D2 dissection is necessary for pT1 (SM) cancer, because the incidence of lymph node metastasis is approximately 10 % and because pT1 (SM) cancer is often accompanied by intermediate lymph node metastasis.
-
(3)
Although there is insufficient evidence of the extent of lymph node dissection for cT2 (MP) cancer, at least D2 dissection is necessary. However, D3 dissection can be performed, because approximately 1 % of cT2 (MP) cancer is accompanied by main lymph node metastases (Table 5) and because preoperative diagnosis of depth of invasion is not very accurate.
Table 5 Lateral lymph node dissection and lateral lymph node metastasis of rectal cancer
-
(1)
-
-
Surgical treatment for rectal cancer:
-
[Indications for lateral lymph node dissection]
-
Lateral lymph node dissection is indicated when the lower border of the tumor is located distal to the peritoneal reflection and the tumor has invaded beyond the muscularis propria [30].
-
-
[Local excision for rectal cancer]
-
Local excision is indicated for cTis (M) cancer and cT1 (SM) cancer (slight invasion) located distal to the second Houston valve (peritoneal reflection).
-
Histological investigation of the resected specimen enables determination of the likelihood that treatment will cure the condition completely, and the need for additional treatment (intestinal resection accompanied by lymph node dissection).
-
-
[Autonomic nerve-preserving surgery]
-
The autonomic nervous system of concern in surgery for rectal cancer comprises the lumbar splanchnic nerves, superior hypogastric plexus, hypogastric nerves, pelvic splanchnic nerves, and pelvic plexus. Taking into consideration such factors as the extent of cancer progression and the presence or absence of macroscopic nerve invasion, preservation of autonomic nerves is attempted to preserve urinary and sexual function as much as possible, if curability is unaffected.
-
-
Laparoscopic surgery:
-
The indications for laparoscopic surgery are determined by considering the surgeon’s experience and skills and characteristics of the tumor, for example the location and extent of progression of the cancer, and patient factors, for example obesity and history of open abdominal surgery (CQ-4).
-
-
Comments
-
[Lateral lymph node dissection]
-
①
An analysis of 2,916 cases of rectal cancer in the project study by the JSCCR showed that the incidence of lateral lymph node metastasis was 20.1 % among patients whose lower tumor border was located distal to the peritoneal reflection and whose cancer invaded beyond the muscularis propria (only patients who underwent lateral lymph node dissection) (Table 5). After performing lateral lymph node dissection for this indication, it is expected that the risk of intrapelvic recurrence decreases by 50 %, and 5-year survival improves by 8 to 9 % [34].
-
②
The incidence of lateral lymph node metastasis was 27 % among patients whose lower tumor border was located distal to the peritoneal reflection and who had lymph node metastasis in the mesorectum.
-
③
Urinary function and male sexual function may be impaired after lateral dissection, even if the autonomic nervous system is completely preserved.
-
①
-
[Aggregate data from the colorectal cancer registry]
-
①
The incidence of lymph node metastasis according to site and depth of tumor invasion, prevalence of curative resection, and 5-year survival is shown in Tables 6, 7, and 8 [29].
Table 6 Incidence of lymph node metastasis according to primary site and depth of tumor invasion Table 7 Curative resection rate according to stage (lower rows: no. of patients) Table 8 Cumulative 5-year survival according to site (lower rows: no. of patients) -
②
Five-year survival after curative resection of pStage 0 to pStage III colorectal cancer according to site was: all sites 82.2 %, colon 83.8 %, rectosigmoid 81.7 %, Ra-Rb rectum 79.3 % (patients in years 2000–2004).
-
①
Chapter 2: Treatment strategies for Stage IV colorectal cancer [26] (Fig. 3)
-
Stage IV colorectal cancer is associated with synchronous distant metastasis to any of the organs: liver, lung, peritoneum, brain, distant lymph nodes, or other organ (e.g., bone, adrenal gland, spleen).
-
If both the distant metastases and the primary tumor are resectable, curative resection of the primary tumor is performed, and resection of the distant metastases is considered.
-
If the distant metastases are resectable but the primary tumor is unresectable, in principle, resection of the primary tumor and distant metastases is not performed, and another treatment method is selected.
-
If the distant metastases are unresectable but the primary tumor is resectable, the indication for resection of the primary tumor is determined on the basis of the clinical symptoms of the primary tumor and the effect on prognosis (CQ-5).
-
Comments
-
①
The incidence of synchronous distant metastasis is shown in Table 9.
Table 9 Incidence of synchronous distant metastasis of colorectal cancer -
②
Distant metastasis associated with peritoneal dissemination (CQ-6).
-
Complete resection is desirable for P1.
-
Complete resection is considered for P2 when easily resectable.
-
The efficacy of resection of P3 has not been demonstrated.
-
-
①
-
③
Cases accompanied by distant metastasis to multiple organs
-
④
Adjuvant therapy subsequent to the resection of distant metastasis
-
The efficacy and safety of adjuvant chemotherapy after resection of distant metastases in colorectal cancer have not been established, and no randomized controlled trials have been implemented regarding whether or not this extends survival [37, 38] (CQ-8). Ideally, appropriately planned clinical trials should be conducted.
-
Chapter 3: Treatment strategies for recurrent colorectal cancer (Fig. 4)
-
The purpose of treatment of recurrent colorectal cancer is improvement of prognosis and the patient’s QOL.
-
Treatment methods include surgery, systemic chemotherapy, arterial infusion chemotherapy, thermal coagulation therapy, and radiotherapy.
-
An appropriate treatment method is selected with the informed consent of the patient, taking into consideration a variety of factors, for example prognosis, complications, and QOL expected after treatment.
-
If recurrence is observed in a single organ and complete surgical resection of the recurrent tumor(s) is possible, resection is strongly considered.
-
If recurrence is observed in more than a single organ, resection can be considered if the recurrent tumors in all of the organs are resectable [35, 39]; however, there is no consensus on the effects of treatment (CQ-7).
-
Some authors believe that resection of liver or lung metastases should be performed only after a specific period of observation to rule out occult metastases [40].
-
Systemic chemotherapy is effective with regard to cases of inoperable liver metastasis, with some cases indicating that curative resection may become possible [41, 42] (CQ-9).
-
Treatment methods for hematogenous metastases are discussed in Chapter 4 “Treatment strategies for hematogenous metastases”).
-
Local recurrences of rectal cancer take the form of anastomotic recurrences and intrapelvic recurrences.
-
(1)
Resection is considered for resectable recurrences.
-
(2)
Radiotherapy and systemic chemotherapy, either alone or in combination, are considered for unresectable recurrences.
-
(1)
-
Comments
-
[Local recurrence of rectal cancer]
-
①
The extent of spread of the recurrent tumor is evaluated by diagnostic imaging, and resection is considered only for patients in whom complete resection can be expected, after taking into consideration such factors as the pattern of recurrence, symptoms, and physical findings (CQ-10).
-
①
Chapter 4: Treatment strategies for hematogenous metastases (Fig. 5)
-
1.
Treatment strategies for liver metastases
-
Treatment of liver metastases is broadly divided into hepatectomy, systemic chemotherapy, hepatic arterial infusion therapy, and thermal coagulation therapy.
-
Hepatectomy is recommended for liver metastases when curative resection is possible.
-
Hepatectomy consists of systematic resection and partial (non-systematic) resection.
-
Indication criteria for hepatectomy
-
(1)
The patient is capable of tolerating surgery.
-
(2)
The primary tumor has been controlled or can be controlled.
-
(3)
The metastatic liver tumor can be completely resected.
-
(4)
There are no extrahepatic metastases or they can be controlled.
-
(5)
The function of the remaining liver will be adequate.
-
Systemic chemotherapy is considered for patients with unresectable liver metastases whose general condition can be maintained at a specific level or higher (PS 0 to PS 2).
-
Thermal coagulation therapy consists of microwave coagulation therapy (MCT) and radiofrequency ablation (RFA).
-
If the patient’s general condition is poor (PS ≥ 3), or there is no effective chemotherapy, best supportive care (BSC) is provided.
-
-
(1)
-
-
Comments
-
[Hepatectomy]
-
①
There is evidence of the efficacy of hepatectomy for patients who have controllable extrahepatic metastases (mainly lung metastases) in addition to liver metastases [35, 36, 39, 43] (CQ-7).
-
②
The efficacy of systemic chemotherapy and hepatic arterial infusion therapy after hepatectomy has not been established (CQ-8).
-
③
The safety of preoperative chemotherapy for resectable liver metastases has not been established (CQ-11).
-
①
-
[Treatment methods other than resection]
-
①
Systemic chemotherapy is performed for patients with unresectable liver metastases (CQ-9).
-
②
In cases of inoperable liver metastasis, the primary lesion should, ideally, be managed if hepatic arterial infusion therapy or heat coagulation therapy is being used (CQ-17, CQ-12).
-
③
Heat coagulation therapy is advantageous in that it is minimally invasive, in addition to having been reported as improving local control and long-term survival in some cases [44, 45]. However, there have not yet been any studies or reports of long-term prognosis involving sufficiently cumulative case studies; consequently, its efficacy has not been established. There is a high incidence of recurrence in comparison with resection, however, and long-term survival is reported to be poor [46], so it is not recommended as an alternative to surgical resection [47] (CQ-12).
-
①
-
2.
Treatment strategies for lung metastases
-
Treatment of lung metastases consists of pneumonectomy and systemic chemotherapy, and radiotherapy.
-
Pneumonectomy is considered if the metastatic lung tumor is resectable.
-
Pneumonectomy consists of systematic resection and partial (non-systematic) resection.
-
-
Indication criteria for pneumonectomy
-
(1)
The patient is capable of tolerating surgery.
-
(2)
The primary tumor has been controlled or can be controlled.
-
(3)
The metastatic lung tumor can be completely resected.
-
(4)
There are no extrapulmonary metastases or they can be controlled.
-
(5)
The function of the remaining lung will be adequate.
-
Systemic chemotherapy is considered for patients with unresectable lung metastases whose general condition can be maintained at a specific level or higher.
-
Even if the patient cannot tolerate surgery, stereotactic body radiotherapy is considered if the primary tumor and extrapulmonary metastases are controlled or can be controlled and the number of lung metastases less than 5 cm in diameter is no more than three [48].
-
If the patient’s general condition is poor, appropriate BSC is provided.
-
-
(1)
-
3.
Treatment strategies for brain metastases
-
Brain metastases are often detected as part of a systemic disease, and surgical therapy or radiotherapy is considered for lesions for which treatment can be expected to be effective.
-
The optimum treatment method is selected after considering the patient’s general condition and status of other metastatic tumors, and after evaluating the size and location of metastatic brain tumors and the number of brain lesions.
-
Radiotherapy is considered for patients with unresectable metastases.
-
-
[Surgical therapy]
-
Indications for brain resection [49]
-
(1)
The patient has a life expectancy of at least several months.
-
(2)
Resection will not cause significant neurological symptoms.
-
(3)
There are no metastases to other organs or they can be controlled.
-
(1)
-
[Radiotherapy]
-
The purpose of radiotherapy is to relieve such symptoms as cranial nerve symptoms and intracranial hypertension symptoms, and to prolong survival time by reducing locoregional relapse.
-
Whole-brain radiotherapy is considered for patients with multiple brain metastases and for patients with a solitary brain metastasis for which surgical resection is not indicated.
-
Stereotactic irradiation is considered when the number of brain metastases is about no more than three or four and the maximum diameter of each metastasis does not exceed 3 cm.
-
-
4.
Treatment strategies for hematogenous metastases to other organs
-
Resection is also considered for other hematogenous metastases, for example the adrenal glands, skin, and spleen, if they are resectable. However, patients with such metastases often have metastasis to more than one organ, and chemotherapy or radiotherapy is often indicated.
-
Chapter 5: Chemotherapy
-
Chemotherapy consists of adjuvant chemotherapy to prevent postoperative recurrence and systemic chemotherapy to treat unresectable colorectal cancer.
-
Commonly used anticancer drugs that have been approved for the indication of colorectal cancer and are covered by Japanese National Health Insurance are:
- Oral drugs:
-
5-FU, tegafur, UFT, doxifluridine (5′-DFUR), carmofur (HCFU), S-1 (S), UFT + leucovorin (LV), capecitabine (Cape), regorafenib, among others
- Injection drugs:
-
5-FU, mitomycin C, irinotecan (IRI), 5-FU + l-leucovorin (l-LV), oxaliplatin (OX), bevacizumab (Bmab), cetuximab (Cmab), panitumumab (Pmab), among others
-
1.
Adjuvant chemotherapy
-
Postoperative adjuvant chemotherapy is systemic chemotherapy that is performed after surgery to prevent recurrence and improve the prognosis of patients who have undergone R0 resection [50].
-
-
General principles of indications for adjuvant chemotherapy
-
(1)
Stage III colorectal cancer (colon and rectal cancer) for which R0 resection has been performed. See CQ-8 for Stage IV resection cases.
-
(2)
The function of major organs is maintained. The following guidelines are provided.
-
Bone marrow: Peripheral blood WBC count >3500/mm3; platelet count >100,000/mm3
-
Liver function: Total bilirubin <2.0 mg/dL; AST/ALT <100 IU/L,
-
Renal function: Serum creatinine concentration no higher than the upper limit of the normal at the institution.
-
-
(3)
Performance status (PS) of 0 or 1.
-
(4)
The patient has recovered from postoperative complications, if any.
-
(5)
The patient has provided written informed consent.
-
(6)
The patient has no serious complications (especially, no intestinal obstruction, diarrhea, or fever).
-
(1)
-
Recommended therapy (listed in the order of the date of their coverage by Japanese National Health Insurance)
-
5-FU + l-LV note
-
UFT + LV
-
Cape
-
FOLFOX
-
CapeOX
-
-
Recommended administration period (CQ-15)
-
In principle, the administration period is 6 months.
- Note:
-
The Roswell Park Memorial Institute (RPMI) method of 5-FU + LV therapy as adjuvant chemotherapy (drip infusion of l-LV 250 mg/m2 administered for 2 h; intravenous infusion of 5-FU 500 mg/m2 slowly administered within 3 min at 1 h after the start of administration of l-LV; once-weekly administration for 6 consecutive weeks followed by a 2-week rest period, 3 cycles every 8 weeks [53])
-
-
2.
Chemotherapy for unresectable unresectable colorectal cancer (Fig. 6)
-
In best supportive care (BSC) without any chemotherapy, median survival time (MST) for patients with unresectable colorectal cancer has been reported to be approximately 8 months. Although their MST has been extended to approximately 2 years as a result of recent chemotherapy, unresectable colorectal cancer is still difficult to cure.
-
The purpose of chemotherapy is to prolong survival time and control symptoms by delaying tumor enlargement.
-
Randomized controlled trials among PS 0 to PS 2 patients have resulted in significantly longer survival time in chemotherapy groups than in the BSC groups that did not receive anticancer drugs [54–56].
-
Initially unresectable colorectal cancer may become resectable after successful chemotherapy.
-
Ideally, patients should be divided into two groups and their treatment policy selected according to whether or not they are appropriate for intensive therapy.
-
Patients not appropriate for intensive therapy are defined according to the two aspects patient factors and tumor-related characteristics. Patient factors include patients with a preference for avoiding the occurrence of serious adverse events or those believed to be unable to withstand OX, IRI, or molecular target drugs during first-line treatment because of severe complications. Tumor-related characteristics includes cases of multiple-organ (or multiple) metastases, in which it is considered unlikely that resection will be possible in the future, or patients determined as having asymptomatic, slow progression (those with limited risk of rapid deterioration).
-
Cmab and Pmab are only used in response to wild-type KRAS.
-
Combination with molecular target drugs, for example Bmab or anti-EGFR antibodies, etc., is recommended, but for patients who are not candidates, chemotherapy alone is conducted.
-
-
General principles underlying the indications for systemic chemotherapy
-
(1)
Clinical diagnosis or histopathological diagnosis has been confirmed.
-
(2)
The metastatic or recurrent tumor can be confirmed by imaging.
-
(3)
Performance status (PS) is 0 to 2.
-
(4)
The function of major organs is maintained (administration guidelines are given as 1–3, below).
-
1
Bone marrow: peripheral blood WBC count >3500/mm3; platelet count >100,000/mm3
-
2
Liver function: total bilirubin <2.0 mg/dL; AST/ALT <100 IU/L
-
3
Renal function: serum creatinine concentration no higher than the upper limit of the normal range at the institution.
-
1
-
(5)
The patient has provided written informed consent.
-
(6)
The patient has no serious complications (especially, no intestinal obstruction, diarrhea, or fever).
-
(1)
-
First-line therapy
-
The following are regimens whose usefulness has been demonstrated in clinical trials and that are available as initial therapy covered by Japanese National Health Insurance.
-
(1)
Patients appropriate for intensive therapy
-
-
(2)
Patients not appropriate for intensive therapy
-
Secondary therapy
-
The following regimens are considered as chemotherapy for 2nd-line treatment (CQ-16).
-
(b)
When the patient has become refractory or intolerant to the first-line regimen, including IRI
-
-
(2)
Patients not appropriate for intensive therapy
-
BSC
-
If possible, consider the regimen judged to be optimum
-
-
3rd-line and thereafter
-
Comments
-
①
Careful attention is required when using IRI to treat patients with constitutional jaundice, such as that caused by Gilbert’s syndrome, or to treat patients with high serum bilirubin values. Relationships between genetic polymorphisms of enzymes that metabolize IRI and toxicity have been suggested (attached Side Memo 2).
-
②
Although hepatic arterial infusion therapy results in a good response for liver metastasis, no survival benefit has been demonstrate in comparison with systemic chemotherapy [93] (CQ-17).
- Note 1:
-
FOLFOX—infusional 5-FU + l-LV + OX
- Note 2:
-
CapeOX—Cape + OX
- Note 3:
-
FOLFIRI—infusional 5-FU + l-LV + IRI
- Note 4:
-
FOLFOXIRI—Infusional 5-FU + l-LV + IRI + OX
- Note 5:
-
IRIS—S-1 + IRI
-
①
Chapter 6: Radiotherapy
-
Radiotherapy is used to treat patients with locally advanced rectal cancer, either as adjuvant therapy after surgery, to prevent recurrence, or before surgery, to reduce tumor volume and preserve the anal sphincter, and also as palliative care to relieve the symptoms and prolong the survival of patients with unresectable colorectal cancer who have symptomatic lesions.
-
1.
Adjuvant radiotherapy
-
Adjuvant radiotherapy is classified into three categories, according to the timing of surgery and radiation therapy: preoperative radiotherapy, intraoperative radiotherapy, and postoperative radiotherapy.
-
The purpose of adjuvant radiotherapy is to improve local control and the survival of rectal cancer patients. The purpose of preoperative radiotherapy includes improving anal sphincter preservation and improving resection rate. However, insufficient evidence of improved survival has been found to make this the objective of adjuvant radiotherapy.
-
Preoperative radiotherapy is indicated for patients with T stage clinically diagnosed as “invasion depth cT3 (SS/A) or deeper or cN-positive”; postoperative radiotherapy is indicated for patients with T stage pathologically diagnosed after surgery as “invasion depth cT3 (SS/A) or deeper or pN-positive, where the existence of a surgical dissection plane positive (RM1) or penetration of the surgical dissection plane by the cancer (RMX) is unclear”; and intraoperative radiotherapy is indicated for “surgical dissection plane positive (RM1) or penetration of the surgical dissection plane by the cancer (RMX) is unclear”.
-
Radiotherapy is delivered with a linear accelerator, with electron beams being used for intraoperative radiotherapy and photon beams for external radiotherapy.
-
-
Comments
-
①
Preoperative radiotherapy (CQ-18)
-
1)
Preoperative radiotherapy has the following advantages: seeding during surgery can be prevented by inactivating lesions with irradiation; a high percentage of tumor cells are normo-oxic and radiosensitive, because blood flow to the tumor is maintained; there is little damage to the digestive tract, because the small bowel is not fixed within the pelvic cavity, thereby resulting in low radiation-induced delayed toxicity, which means a less toxic postoperative setting; improvement in R0 resection and anal sphincter preservation can be expected because of tumor size reduction [94].
-
2)
Preoperative radiotherapy has the following disadvantages: early-stage patients may be subjected to overtreatment and postoperative complications may increase.
-
3)
Twelve phase III clinical trials of preoperative radiotherapy (without chemotherapy) have been reported [94], and in 5 of these trials local control was significantly higher in the group that received preoperative radiotherapy than in the surgery alone group. However, improved survival was observed in 1 trial only [95].
-
4)
Two meta-analyses of radiotherapy revealed improved local control compared with surgery alone, and improved survival in the groups that received doses of 30 Gy or more. However, there is controversy about whether survival is improved [96, 97].
-
5)
Trials of short-course radiotherapy with 5 Gy per fraction have been conducted, mainly in Europe [95, 98]. Because the late effects of radiation depend on fraction size, long-term follow-up for late adverse effects, for example anal dysfunction and bowel dysfunction, is necessary.
-
6)
In the Dutch CKVO 95-04 trial, which compared preoperative radiotherapy (25 Gy delivered in five fractions in 1 week) + TME and TME alone to investigate the significance of adding short-course radiotherapy to TME, 5-year and 10-year local control were significantly higher in the combination therapy group, but 5-year and 10-year survival were not significantly different in the two groups [98–100]. The incidences of sexual dysfunction and bowel dysfunction were higher in the preoperative radiation combination therapy group than in the surgery-alone group [101, 102].
-
7)
The effect of preoperative radiotherapy in reducing the size of the primary tumor may enable sphincter preservation. When the purpose of the preoperative radiotherapy is sphincter preservation, it is desirable to perform surgery after allowing an appropriate period for the tumor to decrease in size (6 to 8 weeks after the completion of radiotherapy) [103].
-
8)
In Europe, four randomized controlled trials, including the EORTC trial, were performed to investigate the usefulness of adding chemotherapy to preoperative radiotherapy. The incidence of acute-phase adverse events was significantly higher in the preoperative chemoradiotherapy groups, but pathologic complete response (pCR) was significantly higher than in the preoperative radiotherapy alone groups. In two trials, the exception being the short-course radiotherapy trials, local recurrence was significantly lower in the preoperative chemoradiotherapy group, and sphincter preservation and survival were not significantly different in the two groups [104–107].
-
9)
In a randomized controlled trial that compared preoperative and postoperative chemoradiotherapy, there was no significant difference in the 5-year survival but local recurrence and incidence of grade 3 or higher adverse events were significantly lower in the preoperative chemoradiotherapy group. Among the patients for whom abdominoperineal resection (APR) was considered necessary at the time of enrollment, the percentage of patients for whom sphincter preservation was possible was significantly higher in the preoperative chemoradiotherapy group [108].
-
10)
A randomized controlled trial of 5-FU versus Cape combination chemotherapy for preoperative chemoradiotherapy indicated that the two drugs had the same level of efficacy and safety [109, 110]. NCCN guidelines allow the use of either 5-FU or Cape as standard combination chemotherapy for preoperative chemoradiotherapy. The indications and use of Cape as an adjuvant therapy for rectal cancer, however, have not been approved for use under health insurance in Japan. It is believed possible to try using it, within an appropriate volume range, and with the permission of the ethics committee, for appropriate selected cases.
-
11)
In randomized controlled trials into the efficacy of adding OX to pyrimidine fluoride as combination chemotherapy for preoperative chemoradiotherapy, OX increased harmful phenomena in three tests and had no efficacy with regard to pCR ratio, localized control ratio, and survival [109, 111–113]; moreover, in one test, although harmful phenomena were no different and no analysis of disease-free survival was conducted at the primary endpoint, the pCR ratio was significantly higher [114].
-
2.
Palliative radiotherapy
-
a.
Intrapelvic lesions (CQ-19)
-
The purpose of palliative radiotherapy for intrapelvic lesions is to relieve symptoms such as pain, hemorrhage, and bowel movement disorders caused by intrapelvic tumors.
-
The target volume includes the tumor that is causing the symptoms.
-
-
①
-
[Dose and fractionation]
-
A total dose of 45 to 50 Gy is administered in 1.8 to 2.0 Gy fractions.
-
Depending on the patient’s general condition, for example performance status, and the severity of the symptoms, radiotherapy may be completed more quickly with a larger fraction size, for example 30 Gy in 10 fractions over 2 weeks.
-
-
b.
Extrapelvic lesions
-
(1)
Bone metastases
-
The purpose of palliative radiotherapy for bone metastases is to achieve pain relief, prevent pathological fractures, and prevent and treat spinal cord paralysis.
-
The target volume includes the metastatic bone lesions causing the symptoms.
-
-
(1)
-
[Dose and fractionation]
-
Local field radiotherapy, for example 30 Gy in 10 fractions and 20 Gy in 5 fractions, is widely performed.
-
-
(2)
Brain metastases
-
Hematogenous metastases are discussed in Chapter 4 “Treatment strategies for hematogenous metastases”.
-
-
[Dose and fractionation]
-
When whole brain radiotherapy is performed, 30 Gy in 10 fractions is the standard treatment. If long-term survival is expected, fractionated radiotherapy, for example 37.5 Gy in 15 fractions and 40 Gy in 20 fractions, is considered.
-
When stereotactic radiosurgery is performed, a peripheral dose of 16 to 25 Gy is delivered in a single fraction.
-
Chapter 7: Palliative care
-
Palliative care is a general term for palliative treatment of a variety of mental and physical symptoms related to cancer.
-
Palliative care extends from the time the cancer is diagnosed until the end stage, and different care should be provided depending on the disease stage and symptoms.
-
In principle, cancer treatment should be performed under conditions in which symptom relief is achieved [115], and palliative care should be started at the same time as surgical treatment and chemotherapy.
-
Palliative care to improve the QOL of patients with end-stage colorectal cancer includes:
-
(1)
Pain relief
-
(2)
Surgical treatment
-
(3)
Chemotherapy
-
(4)
Radiotherapy
-
(5)
Counseling for psychiatric symptoms
-
(1)
Chapter 8: Surveillance after surgery for colorectal cancer
-
1.
Surveillance for recurrence after curability A resection of colorectal cancer
-
(1)
Consideration should be given to periodic endoscopic examination for recurrence at the site of local resection or anastomosis in pStage 0 (pTis (M) cancer) cases. Surveillance for recurrence in other organs is not necessary.
-
(2)
pStage I–pStage III cases should be surveyed for recurrence in the liver, lungs, local area, anastomosis, lymph nodes, peritoneum, etc. The following points should be noted:
-
In principle, the duration of surveillance is 5 years after surgery, but surveillance examinations should be scheduled at shorter intervals during the first 3 years after surgery.
-
It should be noted that there is a higher incidence of lung metastasis and local recurrence in rectal cancer than in colon cancer.
-
As a general rule, the duration of surveillance for anastomotic recurrence is until 3 years after surgery.
-
The following is an example of a surveillance schedule after curative resection of Stage I to Stage III colorectal cancer that was designed on the basis of the results of a retrospective investigation of such factors as the common sites and incidence of recurrence and the efficacy of treatment and clinical practice in Japan (Fig. 7).
-
-
2.
Surveillance after curability B resection of colorectal cancer and after resection of recurrent tumors.
-
(1)
The same surveillance method as for Stage III colorectal cancer is used. It should be noted that recurrence and re-recurrence are common in organs previously operated on.
-
(2)
In cases allocated curability B due to R1 resection, close surveillance schedule should be planned for organs in which residual cancer is suspected.
-
(1)
-
3.
Surveillance of metachronous multiple cancer
-
Colonoscopy is performed for surveillance of metachronous multicentric colorectal cancer.
-
-
Comments
-
①
Purpose of surveillance
-
The purpose of surveillance is to improve the patient’s prognosis by early detection and treatment of recurrences. Meta-analyses of RCTs conducted in Europe and the United States have shown that surveillance after curative surgical resection of colorectal cancer contributes to improving the likelihood of resection of recurrent tumors and to improving the prognosis [116–120] (CQ-20-1).
-
-
①
-
②
Recurrence rate, sites of recurrence, times of recurrence
-
The results of the project study by the JSCCR are shown in Figs. 8, 9 and Tables 10, 11, 12, 13. The subjects were patients who underwent curative resection of colorectal cancer between 1991 and 1996 at the 14 institutions that participated in the project, and the follow-up period was 6–11 years.
Fig. 8 Fig. 9 Table 10 Recurrence after curative resection of colorectal cancer according to stage, and cumulative incidence of recurrence according to number of years after surgery Table 11 Recurrence of Stage I colorectal cancer (RS cancer was counted as colon cancer) Table 12 Recurrence according to site of first recurrence after curative resection of colorectal cancer, and cumulative incidence of recurrence according to number of years after surgery Table 13 Comparison of recurrence of colon cancer and rectal cancer according to the site of the first recurrence (RS cancer was counted as colon cancer)
-
-
(1)
Times and sites of the recurrences (Fig. 9, Tables 10, 12, 13).
-
More than 80 % of the recurrences were detected within 3 years after surgery, and more than 95 % of the recurrences were detected within 5 years after surgery.
-
The overall incidence of recurrence more than 5 years after surgery was less than 1 %.
-
Among lung recurrences, 5 % of recurrences were detected more than 5 years after surgery.
-
More than 95 % of the anastomotic recurrences were detected within 3 years after surgery.
-
Local recurrence and lung recurrence were more frequent for rectal cancer than for colon cancer.
-
There have been reports of recurrence after curative resection in Europe and the United States showing that approximately 50 % of recurrences were detected within 1 year after surgery, that approximately 70 % of the recurrences were detected within 2 years after surgery [121, 122]; and that for most patients recurrence was detected within 5 years after surgery [122].
-
-
(2)
Characteristics of recurrence according to pStage (Fig. 8, Tables 10, 11)
-
1.
pStage I
-
The incidence of recurrence of pT1 (SM) cancer was approximately 1 % for both colon and rectal cancer.
-
Overall recurrence of pT2 (MP) cancer was 6.4 %; it was 5.0 % for colon cancer and 8.3 % for rectal cancer.
-
Two thirds of the recurrences were detected within 3 years after surgery; overall recurrence more than 5 years after surgery was less than 0.2 % among all patients.
-
1.
-
2.
pStage II, pStage IIIa, and pStage IIIb
-
The incidence of recurrence increased with Stage.
-
78 to 90 % of recurrences were detected within 3 years after surgery, and the overall incidence of recurrence more than 5 years after surgery was less than 1 % among all patients.
-
-
③
Surveillance of metachronous multiple primary cancer
-
A past history of colorectal cancer, irrespective of stage, is a risk factor for metachronous colorectal cancer [123].
-
The recommended period between colonoscopy ranged from 1 to 5 years, depending on the report [124].
-
The need for surveillance targeting multiple cancers should be determined by distinguishing hereditary colorectal cancer [125]. There is little evidence of a need for periodic minute examinations for cancer in other organs after surgery for sporadic colorectal cancer (CQ-20-2).
Clinical Questions
CQ-1: What are the indication criteria for additional treatment after endoscopic resection of pT1 (SM) [26]? (Fig. 10)
-
①
Surgical resection is preferable when the vertical margin is positive. (Recommendation/Evidence level 1C)
-
②
If any of the following findings is observed during histological examination of the resected specimen, intestinal resection with lymph node dissection is considered as an additional treatment. (Evidence level B)
-
Note)
-
“Vertical margin-positive” means that carcinoma is exposed at the submucosal margin of the resected specimen.
-
Depth of SM invasion is measured by the method described in Side Memo 1 (Fig. 11).
Fig. 11 Method for measuring depth of SM invasion. a When it is possible to identify or estimate the location of the muscularis mucosae, depth of SM invasion is measured from the lower border of the muscularis mucosae. b, c When it is not possible to identify or estimate the location of the muscularis mucosae, depth of SM invasion is measured from the surface layer of the muscularis mucosae. (b) Sessile lesion; (c) pedunculated lesion. d For pedunculated lesions with a tangled muscularis mucosae, depth of SM invasion is measured as the distance between the point of deepest invasion and the reference line, which is defined as the boundary between the tumor head and the stalk. e Invasion by pedunculated lesions that is limited to within the head is defined as “head invasion”
-
Vascular invasion consists of lymphatic and venous invasion (Figs. 12, 13, 14).
Fig. 12 Fig. 13 -
The method of assessing budding is described in Fig. 15.
-
The principle for treatment of pT1 (SM) carcinomas, which are invasive carcinomas, is intestinal resection with lymph node dissection. However, some pT1 (SM) carcinomas have a very low risk of metastasis, and the purpose of these criteria is to minimize the need for additional resections that eventually result in overtreatment of such patients. Although no diagnostic methods enable prediction of lymph node metastasis (pN) without fail, the risk of metastasis can be used as a basis for determining whether or not to perform additional treatment.
Factors such as the depth of submucosal invasion (SM invasion depth) [127], histological type, for example poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous carcinoma [126], the presence of a poorly-differentiated area and muconodules at the site of deepest invasion, budding, and vascular invasion, have been reported to be risk factors for regional lymph node metastasis by pT1 (SM) carcinoma [126, 128].
These criteria for determining whether additional treatment is indicated were prepared on the basis of 3 criteria for performing additional intestinal resection of pT1 (SM) carcinoma described in the “Japanese Classification of Colorectal Carcinoma” (2nd edition, 1980):
-
(1)
obvious intravascular carcinoma invasion;
-
(2)
poorly differentiated adenocarcinoma or undifferentiated carcinoma; or
-
(3)
massive carcinoma invasion extending to the vicinity of the margin [129].
The description of “massive carcinoma invasion” in the 4th edition of the “Japanese Classification of Colorectal Carcinoma” was revised to a more specific description in the 5th edition (1994): “Invasion deeper than ‘very shallow invasion’ (e.g., invasion exceeding approximately 200 μm to 300 μm)” [130].
Subsequent case series studies in Japan have shown that “200 μm to 300 μm” can be extended to 1000 μm [131]. According to the results of the project study by the JSCCR, the incidence of lymph node metastasis for colorectal carcinoma with an SM invasion depth of 1000 μm or more was 12.5 % (Table 14) [127, 131]. However, not all cases with submucosal invasion deeper than 1,000 μm necessarily require additional surgery. Approximately 90 % of patients with a depth of invasion of 1000 μm or more did not have lymph node metastasis, and it is important to determine whether additional treatment is indicated after sufficiently considering other factors in addition to depth of SM invasion, for example whether other risk factors for lymph node metastasis are present, the physical and social background of the patient, and the patient’s wishes. As consensus has not yet been achieved within the Guideline Committee, indicators of strength of recommendation in the treatment criteria provided above have not been disclosed. Because budding was demonstrated to be an important risk factor for lymph node metastases in the project study by the JSCCR, additional intestinal resection has been added to the list of factors that should be considered according to the previous edition. Furthermore, project research is currently in progress into other histopathological factors. Multi-center joint research projects have produced reports providing results from consideration of the appropriateness of these criteria [132–134]. None of the guidelines in other countries includes depth of invasion or budding as criteria for additional treatment.
CQ-2: What are the criteria for selecting endoscopic resection with regard to lesions with a maximum diameter of 2 cm or greater?
-
Accurate preoperative endoscopic diagnosis is essential in endoscopic resection with regard to lesions with a maximum diameter of 2 cm or greater, and whether resection by EMR, piecemeal EMR, or ESD is indicated is determined after taking the operator’s skill in performing endoscopic resection into consideration. (Recommendation/Evidence level 1B)
-
Side Memo 1
-
■ Method for measuring depth of SM invasion (Fig. 11)
-
When it is possible to identify or estimate the location of the muscularis mucosae, depth of SM invasion is measured from the lower border of the muscularis mucosae of the lesion, irrespective of macroscopic type.
-
When it is not possible to identify or estimate the location of the muscularis mucosae, the depth of SM invasion is measured from the surface of the lesion. The phrase “possible to identify or to estimate” means that there is no “deformity”, i.e., disarray, dissection, rupture, fragmentation, etc., of the muscularis mucosae as a result of SM invasion. If a deformed muscularis mucosa is used as the baseline of the measurement, the depth of SM invasion may be underestimated. Although judging whether there is a “deformity” is not always straightforward, if a desmoplastic reaction is present around the muscularis mucosae, it is assumed to be “deformed.”
-
For pedunculated lesions with a tangled muscularis mucosae, depth of SM invasion is measured as the distance between the point of deepest invasion and the reference line, which is defined as the boundary between the tumor head and the stalk (the boundary between the tumor area and the non-tumor area in the mucosa). Invasion by pedunculated lesions that is limited to within the head is defined as “head invasion.”
-
Attention to arteries is a key factor in assessing venous invasion. Venous invasion is highly likely when a circular, semicircular, or oblong cancer cell nest with regular margins is located in the vicinity of an artery and distant from the main lesion. Such a cancer cell nest surrounded by venous wall structures (for example internal elastic membrane or perivascular smooth muscle) can be regarded as indicative of venous invasion. However, the venous wall structures are often displaced or obliterated by the cancer cell nest, and it is difficult to recognize in hematoxylin and eosin stained sections.
-
The presence of cancer cells and cancer cell nests in the interstitial space suggests lymphatic invasion. A space filled with lymph and lymphocytes is especially likely to be a lymph vessel. When endothelial cells are identified around the space, the space can be regarded as a lymph vessel. However, it is often difficult to identify endothelial cells in specimens stained with hematoxylin and eosin, and spaces may be artifacts created during the process of preparing the specimen.
-
As stated above, evaluation of vascular invasion, which is an important indicator for determining treatment strategies for SM cancer, is often difficult for hematoxylin and eosin stained specimens. Special staining methods are useful for evaluating vascular invasion, for example elastica van Gieson staining or Victoria blue staining for venous invasion, and D2-40 immunostaining for lymphatic invasion.
-
■ Method for the assessing tumor budding (Fig. 15)
[Definition of tumor budding] [126] A cancer cell nest consisting of 1 or less than 5 cells that infiltrates the interstitium at the invasive margin of the cancer.
[Grade of budding] After selecting one field in which the number of budding is greatest, the number of buddings is counted in a field measuring 0.785 mm2 observed through a 20× objective lens (WHK 10× ocular lens). Depending on the number of buddings, grade of budding is defined as:
-
Grade 1: 0 to 4
-
Grade 2: 5 to 9
-
Grade 3: 10 or more
-
The incidence of lymph node metastasis for Grade 2/3 tumors is significantly higher than for Grade 1 tumors. A multi-center study conducted by the Budding Investigation Project Committee (2005–current) of the JSCCR in which Grade 1 was defined as “low grade” and Grade 2/3 as “high grade” showed that “high grade” is an independent predictor of lymph node metastasis.
CQ-3: What cautions should be noted when using colorectal ESD to implement endoscopic resection of colonic lesions?
-
When ESD is being considered for use in cases of “early-stage malignant tumors”, accurate preoperative endoscopic diagnosis and the level of skill of the operator with regard to endoscopic resection should be considered before deciding to proceed. (Recommendation/Evidence level 1B)
CQ-4: Is laparoscopic surgery for colorectal cancer effective?
-
According to randomized controlled trials held overseas and the Cochrane Database of Systematic Reviews, the safety and long-term outcome of laparoscopic surgery for cases of colonic and RS cancers are similar to those for open surgery. Because D3 dissection is difficult under laparoscopic conditions, laparoscopic surgery for cStage II—cStage III disease should be implemented when it is considered that the individual surgical team is sufficiently experienced. Laparoscopic surgery is also difficult for patients with transverse colon cancer, for severely obese patients, and for patients with severe adhesions.
-
The efficacy and safety of laparoscopic surgery for rectal cancer has not been established. Ideally, appropriately planned clinical trials should be implemented. (Recommendation/Evidence level 1B)
CQ-5: Resection of the primary tumor for patients with unresectable distant metastases
-
The efficacy of primary tumor resection for cases with unresectable distant metastases differs depending on such individual factors as symptoms caused by the primary lesion, the state of distant metastasis, the patient’s general condition, etc.
-
①
If symptoms exist, as a result of the primary tumor, which are difficult to control using other therapy, and the resection is not significantly invasive, primary tumor resection and early systemic chemotherapy is recommended. (Recommendation/Evidence level 1C)
-
②
For cases in which no symptoms are caused by the primary tumor, however, the efficacy of resecting the primary tumor has not been established.
CQ-6: In cases where peritoneal dissemination is noted, is it effective to resect peritoneal dissemination at the same time as the primary lesion?
-
The efficacy of resecting peritoneal dissemination has not been proved. Some cases of long-term survival have been reported in which localized dissemination (P1, P2) was resected with the primary tumor, suggesting that if the resection is not significantly invasive peritoneal dissemination should be resected at the same time as the primary tumor. (Recommendation/Evidence level 2D)
CQ-7: What are the indications for resection for cases in which metastasis is simultaneously noted in the liver and the lungs?
-
The efficacy of resection for patients who have liver and lung metastases at the same time has been shown, and thus resection should be considered for patients with resectable liver and lung metastases. However, there are insufficient data to determine the indication criteria for surgery. It is necessary to obtain informed consent after informing the patient of the rather low cure rate and the absence of outcome predictors. (Recommendation/Evidence level 2D).
CQ-8: Is adjuvant chemotherapy effective subsequent to distant metastatic lesion resection?
-
The efficacy and safety of adjuvant chemotherapy subsequent to distant metastatic lesion resection in cases of colorectal cancer have not yet been established. Ideally, appropriately planned clinical trials should be implemented. (Evidence level C)
CQ-9: Is resection of liver/lung metastasis effective, if it becomes possible as a result of the effects of chemotherapy?
-
Resection should be performed for cases in which chemotherapy has successfully made localized metastasis to the liver or lungs operable. (Recommendation/Evidence level 2D)
CQ-10: What are the surgical indications in cases of local recurrence of rectal cancer?
-
Resection should be considered for local recurrence of rectal cancer when R0 resection is considered possible. (Recommendation/Evidence level 2D)
CQ-11: Is preoperative adjuvant chemotherapy effective in cases of operable liver metastasis?
-
The efficacy and safety of preoperative chemotherapy for resectable liver metastases has not been established. It should be evaluated in properly designed clinical trials. (Evidence level D)
CQ-12: Is heat coagulation therapy effective with regard to liver metastatic lesions?
-
①
There are few reports indicating the efficacy of heat coagulation therapy; it is, therefore, not recommended as a first choice of treatment. (Recommendation/Evidence level 1C)
-
②
Because heat coagulation therapy is accompanied by a high risk of local recurrence in cases of liver metastasis, resection should be initially considered wherever possible.
CQ-13: Is postoperative adjuvant chemotherapy effective for patients aged 70 or over?
-
Even for patients 70 years old or older, postoperative adjuvant chemotherapy is recommended if their PS is good, if the function of their major organs is adequate, and if there are no complications that may be a risk for performing chemotherapy. (Recommendation/Evidence level 1A)
CQ-14: Should postoperative adjuvant chemotherapy be conducted for Stage II [26] colorectal cancer?
-
The usefulness of postoperative adjuvant chemotherapy for Stage II colorectal cancer has not been proved, and it is recommended not to routinely administer adjuvant chemotherapy to all patients with Stage II colorectal cancer. (Recommendation/Evidence level 1A)
CQ-15: Is the appropriate duration of postoperative adjuvant chemotherapy 6 months?
-
Although no definitive conclusion regarding the duration of postoperative adjuvant chemotherapy has been reached, the current standard duration of treatment by 5-FU-based adjuvant chemotherapy is 6 months. (Recommendation/Evidence level 1A)
CQ-16-1: Is bevacizumab administration effective as second-line chemotherapy?
-
Combination chemotherapy using bevacizumab is effective as second-line chemotherapy, irrespective of whether bevacizumab was administered as part of initial therapy. (Recommendation/Evidence level 2B)
CQ-16–2: Is administration of molecular target drugs (anti-EGFR antibodies) effective as second-line chemotherapy?
-
For wild-type KRAS cases, treatment with anti-EGFR antibodies (cetuximab and/or panitumumab) is effective. (Recommendation/Evidence level 2C)
Side Memo 2
-
■ IRI and UGT1A1 genetic polymorphism
SN-38 is an active metabolite of IRI and the UGT1A1 gene encodes an intrahepatic metabolizing enzyme which converts the active form SN-38 to the inactive form SN-38 G. Among patients who are double heterozygotes for *6 and *28 or homozygotes for *6 or *28 of the UGT1A1 gene, the glucuronic acid conjugation capacity of UGT1A1 is known to be reduced and metabolism of SN-38 to be delayed, and serious adverse drug reactions, for example neutropenia, may occur as a result. It is especially desirable to test for a UGT1A1 genetic polymorphism before administering IRI to patients with a high serum bilirubin level, elderly patients, patients whose general condition is poor (e.g., PS2), and patients for whom severe toxicity (especially neutropenia) developed after the last administration of IRI. On the other hand, because IRI toxicity cannot be predicted with certainty on the basis of the presence of a UGT1A1 genetic polymorphism alone, it is essential to monitor patients’ general condition during treatment and to manage adverse drug reactions carefully, irrespective of whether a genetic polymorphism is detected.
CQ-17: Is hepatic arterial infusion therapy effective in cases of liver metastases?
-
Comparisons between hepatic arterial infusion therapy using fluoropyrimidine alone and systemic chemotherapy showed no clear difference in survival. The effectiveness of hepatic arterial infusion therapy in comparison with systemic chemotherapy using multi-drug combination has not been established. (Recommendation/Evidence level 1C)
CQ-18: Is preoperative chemoradiotherapy effective in patients with rectal cancer?
-
In the USA and Europe, although preoperative chemoradiotherapy has reduced the incidence of local recurrence in comparison with TME-only, reports suggest that it has not contributed to improved survival. In Japan, where surgical methods differ from the USA and Europe, the efficacy of preoperative chemoradiotherapy has not been established with regard to rectal cancers for which the lower margin of the tumor is closer to the anus than the peritoneal reflection. (Evidence level B)
CQ-19: Is chemoradiotherapy effective for unresectable locally advanced and locally recurrent rectal cancer?
-
①
In cases of locally advanced and locally recurrent rectal cancer determined likely to become R0 resectable as a result of tumor shrinkage after treatment, it is recommended that chemoradiotherapy, with the objective of resection, be used as opposed to radiotherapy alone. (Recommendation/Evidence level 1B)
-
②
Chemoradiotherapy should also be taken into consideration where the objective is relief of symptoms. (Recommendation/Evidence level 1C)
CQ-20-1: Is surveillance subsequent to curative surgery for colorectal cancer effective?
-
It has been suggested that the efficacy of surveillance is its contribution to improving prognosis by enabling early detection of recurrence, and, as such, regular postoperative surveillance is desirable. (Recommendation/Evidence level 1A)
-
However, an optimum surveillance protocol incorporating a health-economical perspective has not been sufficiently established.
CQ-20-2: Is surveillance of multiple cancers (multiple colorectal cancer or other organ cancer) effective subsequent to curative surgery for colorectal cancer?
-
①
Metachronous colorectal cancer occurs more frequently in cases of colorectal cancer resection than in the general population, and, as such, regular endoscopic examination of the colon is recommended. (Recommendation/Evidence level 1B)
-
②
There is no indication that post-surgical surveillance targeting multiple cancers is effective. The appropriate course of action is to educate the patient regarding the need for regular cancer examinations and recommend periodic checkups. (Recommendation/Evidence level 2C)
References
Japanese Society for Cancer of the Colon and Rectum (2014) JSCCR Guidelines 2014 for the Treatment of Colorectal Cancer. Kanehara and Co. Ltd, Tokyo
Fukui T, Yamaguchi N, Morizane T et al (2014) Minds handbook for clinical practice guideline development 2014. Igaku Shoin, Tokyo
Aihara M, Mihara H, Murayama T et al (2010) GRADE System for clinical practice guideline—therapeutic intervention. Toppan Media, Hirosaki
Atkins D, Best D, Briss PA et al (2004) The GRADE* Working Group: Grading quality of evidence and strength of recommendations. BMJ 328:1490–1494 printed, abridged version
Guyatt GH, Oxman AD, Vist G et al (2008) GRADE Working Group: Rating quality of evidence and strength of recommendations. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Guyatt GH, Oxman AD, Kunz R et al (2008) GRADE Working Group: Rating quality of evidence and strength of recommendations: what is “quality of evidence” and why is it important to clinicians? BMJ 336:995–998
Schunemann HJ, Oxman AD, Brozek J et al (2008) GRADE Working Group: Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 336:1106–1110
Guyatt GH, Oxman AD, Kunz R et al (2008) GRADE Working Group: Rating quality of evidence and strength of recommendations: Incorporating considerations of resources use into grading recommendations. BMJ 336:1170–1173
Guyatt GH, Oxman AD, Kunz R et al (2008) GRADE Working Group: Rating quality of evidence and strength of recommendations: going from evidence to recommendations. BMJ 336:1049–1051
Jaeschke R, Guyatt GH, Dellinger P et al (2008) GRADE Working Group. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ 337:a744
Guyatt G, Oxman AD, Akl EA et al (2011) GRADE guidelines: 1. Introduction–GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 64:395–400
Balshem H, Helfand M, Schunemann HJ et al (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Guyatt GH, Oxman AD, Vist G et al (2011) GRADE guidelines: 4. Rating the quality of evidence––study limitations (risk of bias). J Clin Epidemiol 64:407–415
Guyatt GH, Oxman AD, Montori V et al (2011) GRADE guidelines: 5. Rating the quality of evidence––publication bias. J Clin Epidemiol 64:1277–1282
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 6. Rating the quality of evidence imprecision. J Clin Epidemiol 64:1283–1293
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE Working Group: GRADE guidelines: 7. Rating the quality of evidence––inconsistency. J Clin Epidemiol 64:1294–1302
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence––indirectness. J Clin Epidemiol 64:1303–1310
Guyatt GH, Oxman AD, Sultan S et al (2011) GRADE Working Group. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 64:1311–1316
Brunetti M, Shemilt I, Pregno S et al (2013) GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol 66:140–150
Guyatt G, Oxman AD, Sultan S et al (2013) GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 66:151–157
Guyatt G, Oxman AD, Santesso N et al (2013) GRADE guidelines: 12. Preparing summary of findings tables–binary outcomes. J Clin Epidemiol 66:158–172
Thorlund K, Oxman AD, Walter SD et al (2013) GRADE guidelines: 13. Preparing summary of findings tables-continuous outcomes. J Clin Epidemiol 66:173–183
Andrews J, Guyatt G, Oxman AD et al (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations J. Clin Epidemiol 66:719–725
Andrews JC, Schunemann HJ, Oxman AD et al (2013) GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. J Clin Epidemiol 66:726–735
Japanese Society for Cancer of the Colon and Rectum (2013) Japanese classification of colorectal carcinoma. The 8th Edition. Kanehara & CO., LTD. Tokyo
Tanaka S, Oka S, Chayama K (2008) Colorectal endoscopic submucosal dissection (ESD): the present status and future perspective including its differentiation from endoscopic mucosal resection (EMR). J Gastroenterol 43:641–651
Kudo S (1993) Endoscopic mucosal resection of flat and depressed early colorectal cancer. Endoscopy 25:455–461
Japanese Society for Cancer of the Colon and Rectum: Multi-institutional registry of large bowel cancer in Japan, Cases treated in 2000–2002, vol. 29 (2011), Cases treated in 2003–2004, vol. 30 (2012)
Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
MacFarlane JK, Ryall RD, Heald RJ (1993) Mesorectal excision for rectal cancer. Lancet 341:457–460
Enker WE, Thaler HT, Cranor ML et al (1995) Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg 181:335–346
Lowry AC, Simmang CL, Boulos P et al (2001) Consensus statement of definitions for anorectal physiology and rectal cancer. Dis Colon Rectum 44:915–919
Sugihara K, Kobayashi H, Kato T et al (2006) Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum 49:1663–1672
Murata S, Moriya Y, Akasu T et al (1998) Resection of both hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer 83:1086–1093
Kobayashi K, Kawamura M, Ishihara T (1999) Surgical treatment for both pulmonary and hepatic metastases from colorectal cancer. J Thorac Cardiovasc Surg 118:1090–1096
Portier G, Elias D, Bouche O et al (2006) Multicentric randomized trial of adjuvant fluorouracil and folnic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 24:4976–4982
Mitry E, Fields AL, Bleiberg H et al (2008) Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 26:4906–4911
Robinson BJ, Rice TW, Strong SA et al (1999) Is resection of pulmonary and hepatic metastases warranted in patients with colorectal cancer? J Thorac Cardiovasc Surg 117:66–76
Lambert LA, Colacchio TA, Barth RJ Jr (2000) Interval hepatic resection of colorectal metastases improves patient selection. Arch Surg 135:473–479
Adam R (2007) Developing strategies for liver metastases from colorectal cancer. Semin Oncol 34(2 Suppl 1):S7–S11
Lam VW, Spiro C, Laurence JM et al (2012) A systematic review of clinical response and survival outcomes of downsizing systemic chemotherapy and rescue liver surgery in patients with initially unresectable colorectal liver metastases. Ann Surg Oncol 19:1292–1301
Regnard JF, Grunenwald D, Spaggiari L et al (1998) Surgical treatment for hepatic and pulmonary metastases from colorectal cancers. Ann Thorac Surg 66:214–218
Beppu T, Matsuda T, Maeda K et al (2000) Microwave coagulation therapy for metastatic liver tumor. Surg Therapy 83:237–242
Shibata T, Niinobu T, Ogata N et al (2000) Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 89:276–284
Park IJ, Kim HC, Yu CS et al (2008) Radiofrequency ablation for metachronous liver metastasis from colorectal cancer after curative surgery. Ann Surg Oncol 15:227–232
Wong SL, Mangu PB, Choti MA et al (2010) American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 28:493–508
Tree AC, Khoo VS, Eeles RA et al (2013) Stereotactic body radiotherapy for oligometastases. Lancet Oncol 14:e28–e37
Patchell RA, Tibbs PA, Walsh JW et al (1990) A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494–500
National Institute of Health Consensus Conference (1990) Adjuvant therapy for patients with colon and rectal cancer. JAMA 264:1444–1450
Benson AB 3rd, Schrag D, Somerfield MR et al (2004) American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 22:3408
Schmoll HJ, Van Cutsem E, Stein A et al (2012) ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 23:2479–2516
Haller DG, Catalano PJ, Macdonald JS et al (2005) Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 23:8671–8678
Scheithauer W, Rosen H, Kornek GV et al (1993) Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. Br Med J 306:752–755
Simmonds PC (2000) Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. Br Med J 321:531–535
Cunningham D, Pyrhonen S, James RD et al (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352:1413–1418
de Gramont A, Figer A, Seymour M et al (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18:2938–2947
Goldberg R, Sargent D, Morton R et al (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22:23–30
Saltz LB, Clarke S, Díaz-Rubio E et al (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 26:2013–2019
Cassidy J, Clarke S, Díaz-Rubio E et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26:2006–2012
Tournigand C, André T, Achille E et al (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22:229–237
Douillard JY, Cunningham D, Roth AD et al (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355:1041–1047
Fuchs CS, Marshall J, Mitchell E et al (2007) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC–C Study. J Clin Oncol 25:4779–4786
Fuchs CS, Marshall J, Barrueco J et al (2008) Randomized, controlled trial of irinotecan plus infusional, bolus or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC–C study. J Clin Oncol 26:689–690
Bokemeyer C, Bondarenko I, Makhson A et al (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671
Douillard JY, Siena S, Cassidy J et al (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin(FOLFOX4)versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28:4697–4705
Van Cutsem E, Köhne CH, Hitre E et al (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Köhne CH, Hofheinz R, Mineur L et al (2012) First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol 138:65–72
Falcone A, Ricci S, Brunetti I et al (2007) Gruppo Oncologico Nord Ovest: Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan(FOLFOXIRI)compared with infusional fluorouracil, leucovorin, and irinotecan(FOLFIRI)as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 25:1670–1676
Petrelli N, Herrera L, Rustum Y et al (1987) A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol 5:1559–1565
de Gramont A, Bosset JF, Milan C et al (1997) Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 15:808–815
Hurwitz H, Fehrenbacher L, Hainsworth JD et al (2005) Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 23:3502–3508
Kabbinavar F, Hurwitz HI, Fehrenbacher L et al (2003) Phase II, randomized trial comparing Bevacizumab plus fluorouracil(FU)/leucovorin(LV)with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21:60–65
Hoff PM, Ansari R, Batist G et al (2001) Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 19:2282–2292
Van Cutsem E, Twelves C, Cassidy J et al (2001) Xeloda Colorectal Cancer Study Group: Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol 19:4097–4106
Tebbutt NC, Wilson K, Gebski VJ et al (2010) Capecitabine, bevacizumab, and mitomycin in first–line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol 28:3191–3198
Shirao K, Hoff PM, Ohtsu A et al (2004) Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur(UFT)plus oral leucovorin(LV)regimen between Japanese and American patients with advanced colorectal cancer: joint United States and Japan study of UFT/LV. J Clin Oncol 22:3466–3474
Douillard JY, Hoff PM, Skillings JR et al (2002) Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20:3605–3616
Carmichael J, Popiela T, Radstone D et al (2002) Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 20:3617–3627
Bennouna J, Sastre J, Arnold D et al (2013) ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomized phase 3 trial. Lancet Oncol 14:29–37
Muro K, Boku N, Shimada Y et al (2010) Irinotecan plus S-1 (IRIS)versus fluorouracil and folinic acid plus irinotecan (FOLFIRI)as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11:853–860
Sobrero AF, Maurel J, Fehrenbacher L et al (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319
Peeters M, Price TJ, Hotko YS, et al (2010) Randomized phase III study of panitumumab (pmab) with FOLFIRI vs FOLFIRI alone as second-line treatment (tx) in patients (pts) with metastatic colorectal cancer (mCRC): Patient-reported outcomes (PRO). 2010 ASCO Gastrointestinal Cancers Symposium. (Abstr 282)
Rothenberg ML, Oza AM, Bigelow RH et al (2003) Superiority of oxaliplatin and fluorouracil–leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil–leucovorin: interim results of a phase III trial. J Clin Oncol 21:2059–2069
Giantonio BJ, Catalano PJ, Meropol NJ et al (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25:1539–1544
Rothenberg ML, Cox JV, Butts C et al (2008) Capecitabine plus oxaliplatin (XELOX)versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4)as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19:1720–1726
Cunningham D, Humblet Y, Siena S et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Jonker DJ, O’Callaghan CJ, Karapetis CS et al (2007) Cetuximab for the treatment of colorectal cancer. N Engl J Med 357:2040–2048
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy–refractory metastatic colorectal cancer. J Clin Oncol 25:1658–1664
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26:1626–1634
Grothey A, Van Cutsem E, Sobrero A et al (2013) CORRECT Study Group: Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 381:303–312
Meta-Analysis Group in Cancer (1996) Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 88:252–258
Skibber JM, Hoff PM, Minsky BD et al (2001) Cancer of the rectum. In: Devita VT, Hellman S, Rosenberg SA (eds) Cancer: principles and practice of oncology, 6th edn. Lippincott, Williams and Wilkins, Philadelphia, pp 1271–1318
Swedish Rectal Cancer Trial (1997) Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 336:980–987
Camma C, Giunta M, Fiorica F et al (2000) Preoperative radiotherapy for resectable rectal cancer. A meta-analysis. J Am Med Assoc 284:1008–1015
Colorectal Cancer Collaborative Group (2001) Adjuvant radiotherapy for rectal cancer: a systematic overview of 22 randomised trials involving 8507 patients. Lancet 358:1291–1304
Kapiteijn E, Marijnen CA, Nagtegaal ID et al (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646
Peeters KC, Marijnen CA, Nagtegaal ID et al (2007) The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 246:693–701
van Gijn W, Marijnen CA, Nagtegaal ID et al (2011) Dutch Colorectal Cancer Group: Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582
Marijnen CA, van de Velde CJ, Putter H et al (2005) Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol 23:1847–1858
Peeters KC, van de Velde CJ, Leer JW et al (2005) Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients: a Dutch colorectal cancer group study. J Clin Oncol 23:6199–6206
Francois Y, Nemoz CJ, Baulieux J et al (1999) Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: The Lyon R90-01 randomized trial. J Clin Oncol 17:2396–2402
Bosset JF, Collette L, Calais G et al: EORTC Radiotherapy Group Trial 22921 (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123
Gerard JP, Conroy T, Bonnetain F et al (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24:4620–4625
Bujko K, Nowacki MP, Nasierowska-Guttmejer A et al (2006) Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 93:1215–1223
Ngan SY, Burmeister B, Fisher RJ et al. (2012) Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 30:3827–3833
Sauer R, Becker H, Hohenberger W, et al; German Rectal Cancer Study Group (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351: 1731–1740
Hofheinz RD, Wenz F, Post S et al (2012) Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 13:579–588
Roh MS, Yothers GA, O’Connell MJ et al (2011) The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R–04. J Clin Oncol (Meeting Abstracts) 29:3503
Aschele C, Cionini L, Lonardi S et al (2011) Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR–01 randomized phase III trial. J Clin Oncol 29:2773–2780
Gérard JP, Azria D, Gourgou-Bourgade S et al (2010) Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405–Prodige 2. J Clin Oncol 28:1638–1644
Gérard JP, Azria D, Gourgou-Bourgade S et al (2012) Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 30:4558–4565
Rödel C, Liersch T, Becker H et al (2012) German Rectal Cancer Study Group: Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO–04 randomised phase 3 trial. Lancet Oncol 13:679–687
Cancer pain relief and palliative care (1990) Report of a WHO expert committee. WHO Technical Report Series 804. WHO, Geneva, 21–22
Renehan AG, Egger M, Saunders MP et al (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trial. Br Med J 324:813–816
Figueredo A, Rumble RB, Maroun J et al (2003) Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 6:26
Renehan AG, Egger M, Saunders MP et al (2005) Mechanisms of improved survival from intensive followup in colorectal cancer: a hypothesis. Br J Cancer 92:430–433
Jeffery M, Hickey BE, Hider PN (2007) Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 24: CD002200
Tjandra JJ, Chan MK (2007) Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 50:1783–1799
Bruinvels D, Stiggelbout A, Kievit J et al (1994) Follow-up of patients with colorectal cancer. A meta-analysis. Ann Surg 219:174–182
Fleischer D, Goldberg S, Browning T et al (1989) Detection and surveillance of colorectal cancer. JAMA 261:580–585
Green RJ, Metlay JP, Propert K et al (2002) Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Int Med 136:261–269
Berman J, Cheung R, Weiberg D (2000) Surveillance after colorectal cancer resection. Lancet 355:395–399
Japanese Society for Cancer of the Colon and Rectum (2012) JSCCR Guidelines 2012 for the Clinical Practice of Hereditary Colorectal Cancer. Kanehara & CO., LTD. Tokyo
Ueno H, Mochizuki H, Hashiguchi Y et al (2004) Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 127:385–394
Kitajima K, Fujimori T, Fujii S et al (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39:534–543
Tanaka S, Haruma K, Oh-e H et al (2000) Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep 7:783–788
Japanese Society for Cancer of the Colon and Rectum (1980) General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus. The 2nd Edition. Kanehara & CO., LTD. Tokyo
Japanese Society for Cancer of the Colon and Rectum (1994) General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus. The 5th Edition. Kanehara & CO., LTD. Tokyo
Japanese Society for Cancer of the Colon and Rectum (2005) JSCCR Guidelines 2005 for the Treatment of Colorectal Cancer. Kanehara & CO., LTD. Tokyo
Oka S, Tanaka S, Kanao H et al (2011) Mid-term prognosis after endoscopic resection for submucosal colorectal carcinoma: summary of a multicenter questionnaire survey conducted by the colorectal endoscopic resection standardization implementation working group in Japanese Society for Cancer of the Colon and Rectum. Dig Endosc 23:190–194
Matsuda T, Fukuzawa M, Uraoka T et al (2011) Risk of lymph node metastasis in patients with pedunculated type early invasive colorectal cancer: a retrospective multicenter study. Cancer Sci 102:1693–1697
Ikematsu H, Yoda Y, Matsuda T et al (2013) Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 144:551–559
Conflict of interest
Toshiaki Watanabe received research grants from Chugai, Taiho, Yakult, Takeda, Merck Serono, and Bristol-Myers Squibb, and received lecture fees from Chugai, Taiho, Yakult Takeda, Merck Serono, Bristol-Myers Squibb, and Bayel; Michio Itabashi has received lecture fees from Chugai; Yasuhiro Shimada received research grants from Taiho, Chugai, Yakult, and Bayer, and received lecture fees from Taiho, Chugai, Yakult, and Bayer; Shinji Tanaka has no conflict of interest; Yoshinori Ito has no conflict of interest; Yoichi Ajioka has no conflict of interest; Tetsuya Hamaguchi serves as a consultant to Takeda; Ichinosuke Hyodo received research grants from Taiho, Chugai, and Yakult, and received lecture fees from Taiho, Chugai, and Yakult; Masahiro Igarashi has no conflict of interest; Hideyuki Ishida has no conflict of interest; Soichiro Ishihara has no conflict of interest; Megumi Ishiguro serves as a consultant to Taiho, and received a research grant from Taiho, and received lecture fees from Taiho, Chugai, Yakult, and Nippon Kayaku, and received writing fees from Taiho; Yukihide Kanemitsu has no conflict of interest; Norihiro Kokudo received research grants from Dainippon Sumitomo, Bayer, Merk-Serono, Bristol-Mayers, Chigai, and Taiho; Kei Muro received lecture fees from Merk Serono, Taiho, Chugai, and Takeda; Atsushi Ochiai has no conflict of interest; Masahiko Oguchi has no conflict of interest; Yasuo Ohkura has no conflict of interest; Yutaka Saito has no conflict of interest; Yoshiharu Sakai has no conflict of interest; Hideki Ueno has no conflict of interest; Takayuki Yoshino received research grants from Daiich Sankyo, Taiho, Eli Lilly, Pfizer, Yakult, and Dainippon Sumitomo, and received lecture fees from Chugai and Takeda; Narikazu Boku received research grants from Taiho and Chugai, and received lecture fees from Taiho and Ono; Takahiro Fujimori has no conflict of interest; Nobuo Koinuma has no conflict of interest; Takayuki Morita has no conflict of interest; Genichi Nishimura has no conflict of interest; Yuh Sakata received lecture fees from Taiho, Daiich Sankyo, Yakult, Otsuka Pharmaceutical, Merck Serono, and Bristol-Myers Squibb, and received manuscript fees from Synergy International; Keiichi Takahashi has no conflict of interest; Osamu Tsuruta has no conflict of interest; Toshiharu Yamaguchi has no conflict of interest; Masahiro Yoshida has no conflict of interest; Naohiko Yamaguchi has no conflict of interest; Kenjiro Kotake has no conflict of interest; Kenichi Sugihara received research grants from Chugai, Taiho, Yakult, Takeda, Merck Serono, Bristol-Myers Squibb, and Bayel, and received lecture fees from Chugai, Taiho, Yakult, Takeda, Merck Serono, Bristol-Myers Squibb, and Bayel, and received manuscript fees from Chugai, Taiho, Merck Serono, Bristol-Myers Squibb, and Bayel.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Watanabe, T., Itabashi, M., Shimada, Y. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 20, 207–239 (2015). https://doi.org/10.1007/s10147-015-0801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-015-0801-z